J Dent Res Dent Clin Dent Prospects. 17(1):1-7.
doi: 10.34172/joddd.2023.36851
Reviews
Influence of dairy products consumption on oral cancer risk: A meta-analysis
Alberto Rodriguez-Archilla Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, 1, * 
Marina Gomez-Fernandez Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, 1
Author information:
Department of Stomatology, Oral Medicine Unit. Faculty of Dentistry. University of Granada, Granada, Spain
Abstract
Background.
The role of dairy product consumption on oral cancer risk is not yet fully clarified. Some studies have observed an inverse association between dairy consumption and oral cancer risk. This study aimed to determine the influence of dairy product consumption (milk, cheese, yogurt, butter) on oral cancer risk.
Methods.
A search for studies on dairy products and oral cancer was conducted in the following databases: PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. The estimation of the odds ratio (OR) effect was performed with the generic inverse variance method using the logarithm of the effect with the standard error (SE) and 95% confidence intervals.
Results.
Twenty-one studies with 59271 participants (8,300 oral cancer patients and 50971 controls) were included in this meta-analysis. All dairy products significantly reduced oral cancer risk except butter (P=0.16). Milk intake reduced oral cancer risk by 27% (OR: 0.73; P<0.001); yogurt consumption by 25% (OR: 0.75; P<0.001), and cheese consumption by 21% (OR:0.79; P<0.01).
Conclusion.
Regular consumption of dairy products reduces oral cancer risk between 21% and 27%.
Keywords: Butter, Cheese, Dairy products, Milk, Mouth neoplasms, Yogurt
Copyright and License Information
©2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Introduction
Oral cancer is the eighth most common cancer in men worldwide, being oral squamous cell carcinoma (OSCC) as the most common histological type (90% of cases). The main oral cancer risk factors are tobacco consumption, alcohol intake, betel nut use, genetic factors, HPV infection, chronic oral mucosal trauma together with poor oral hygiene, and diet.
1
Dietary factors have been related to oral cancer risk, especially the consumption of tea or coffee, fruits, vegetables, or meat. However, there are few studies on dairy product consumption and oral cancer risk with inconclusive results. The influence of dairy product consumption on oral cancer risk is still not fully understood.
2
Apart from insulin-like growth factor (IGF) and calcium, which are risk factors for prostate cancer; milk lipids and fatty acids (linoleic acid, butyric acid, phospholipids, and sphingolipids), are probably beneficial agents against cancer. Milk contains high-quality protein, which can improve immunity and promote the body to recover health. Drinking milk and consuming dairy products may protect against oral cancer.
3
Some studies have found an inverse association between dairy product consumption and oral cancer risk.
4
This study aimed to determine the influence of the consumption of dairy products (milk, cheese, yogurt, butter) on oral cancer risk.
Methods
All research steps (search, study selection, and data extraction) were achieved independently by both authors (ARA and MGF). Discrepancies in article selection were resolved by consensus.
Search strategy
A search for studies on the influence of dairy products (milk, cheese, yogurt, butter) consumption on oral cancer risk up to October 2022 was performed in the following databases: PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. The search strategies in each database using a combination of Medical Subjects Headings (MeSH) and free-text terms are shown in Table 1. The inclusion criteria were as follows: (a) all types of articles related to our purpose, (b) articles without relevant risk of bias (score ≥ 6 stars on the Newcastle-Ottawa methodological quality assessment scale),
5
and (c) articles written in any language and with no restrictions on publication date. The exclusion criteria were: (a) articles with no full-text availability, (b) articles with no clinical data, and (c) studies with non-usable data.
Table 1.
Search strategies for the three databases
|
Database
|
#
|
Search strategy
|
Results
|
| PubMed |
#1 |
“dairy products”[MeSH Terms] OR “milk”[All Fields] |
173,518 |
|
|
#2 |
“mouth neoplasms”[MeSH Terms] OR “oral cancer”[All Fields] |
80,414 |
|
|
#3 |
#1 AND #2 |
65 |
| Web of Science (WoS) |
#4 |
("dairy products"[Topic] OR "milk"[Topic]) |
433,524 |
|
|
#5 |
("mouth neoplasms"[Topic] OR "oral cancer"[Topic]) |
51,243 |
|
|
#6 |
#4 AND #5 |
73 |
| Scopus |
#7 |
TITLE-ABS-KEY ("dairy products" OR "milk") |
256,910 |
|
|
#8 |
TITLE-ABS-KEY ("mouth neoplasms" OR "oral cancer") |
46,428 |
|
|
#9 |
#7 AND #8 |
59 |
Assessment of methodological quality
The methodological quality of the articles was screened using the Newcastle-Ottawa Scale (NOS) methodological quality assessment scale composed of eight items that evaluate three dimensions (selection, comparability, and exposure). Considering the score obtained, the studies are classified as high quality ( ≥ 7 stars), moderate quality (4-6 stars), and low quality (1-3 stars).
Statistic analysis
Data were meta-analyzed with the RevMan 5.4 program (The Cochrane Collaboration, Oxford, UK). The estimation of the odds ratio (OR) effect was conducted with the generic inverse variance method, using the logarithm of the effect with the standard error (SE) and 95% confidence intervals (95% CI). Heterogeneity was determined according to the Higgins statistic (I2). In cases of high heterogeneity (I2 > 50%), the random-effects model was applied. The minimum level of significance was set at P < 0.05.
Results
Study selection
The search found 197 articles (65 in PubMed, 73 in WoS, and 59 in Scopus) between the years 1977 and 2021, 49 of them duplicates, leaving 148 articles for eligibility. 127 articles were excluded due to: (a) articles with no full-text availability (n = 36), (b) articles without clinical data (n = 21), and (c) studies with non-usable data (n = 70). Finally, 21 studies were included in this meta-analysis (Figure 1).
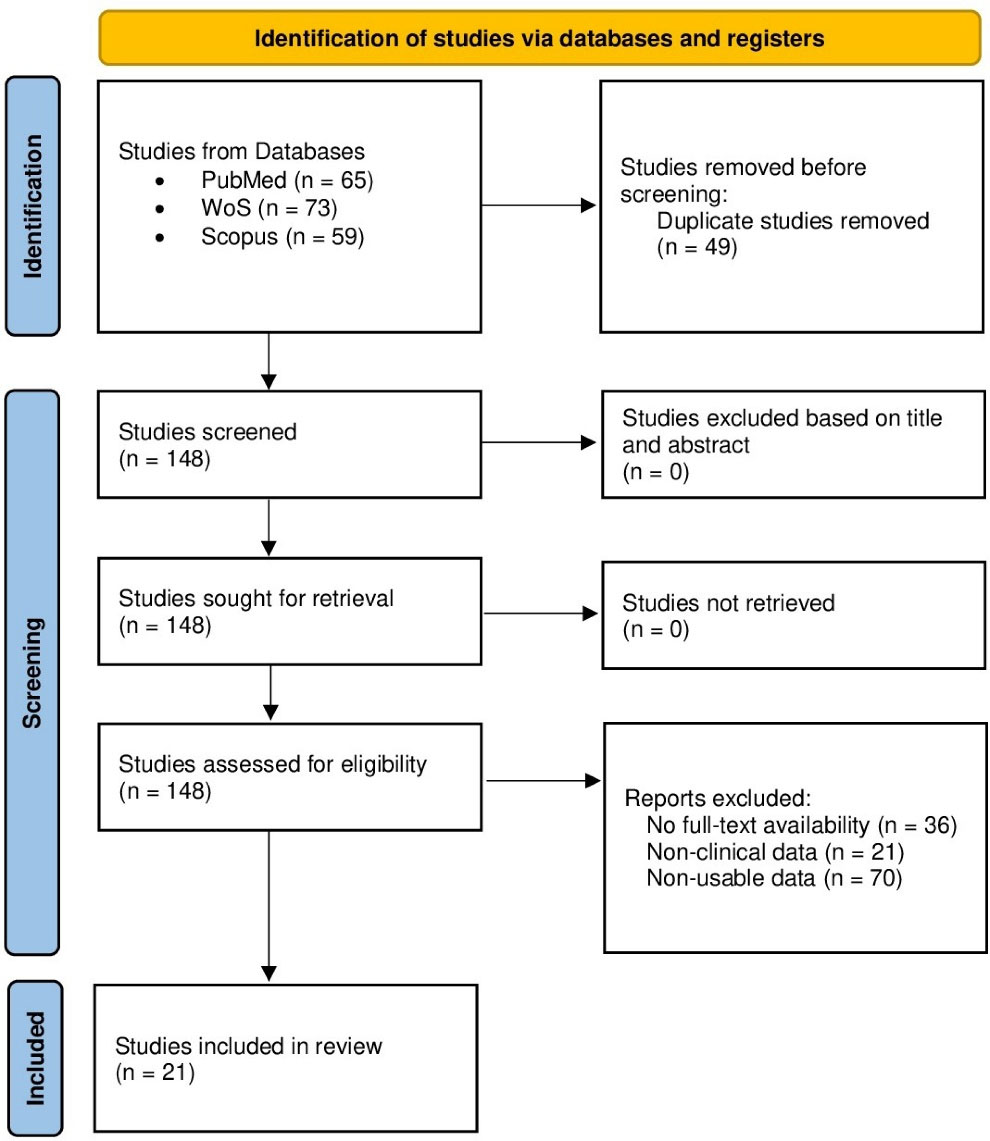
Figure 1.
Study selection flowchart
.
Study selection flowchart
Table 2 presents the main descriptive characteristics and the methodological quality according to the NOS scale of the twenty-one studies
6-26
included in this meta-analysis. A total of 59 271 participants (8300 oral cancer patients and 50 971 controls) were considered. By gender, 23 551 were males (41.2%), and 33 384 were females (58.8%). The studies were conducted in the following countries: Italy (3 studies), the United States of America (3 studies), Brazil (3 studies), India (2 studies), China (2 studies), Switzerland (2 studies), Japan (1 study), Cuba (1 study), Uruguay (1 study), Greece (1 study), Spain (1 study), and Poland (1 study). Considering the NOS quality scale, four articles (19.0%) had 6 stars, fourteen articles (66.7%) got 7 stars, and three articles (14.3%) reached 8 stars.
Table 2.
Characteristics and methodological quality evaluation of the twenty-one articles included in this meta-analysis
|
Study, year
|
Country
|
Study population
|
Dairy product
|
Other parameters analyzed
|
NOS
|
|
Notani, 1987
6
|
India |
278 OC (na, na; na)
215 CS (na, na; na)
|
Milk |
Tobacco, food groups |
7 |
|
La Vecchia, 1991
7
|
Italy |
105 OC (89 M, 16 F; 37-74 y)
1169 CS (875 M, 294 F; 21-74 y)
|
Milk, cheese, butter |
Age, gender, tobacco |
6 |
|
De Stefani, 1994
8
|
Uruguay |
246 OC (246 M, 0 F; 40-89 y)
253 CS (253 M, 0 F; 40-89 y)
|
Milk |
Age, gender, tobacco, alcohol, food groups |
6 |
|
Takezaki, 1996
9
|
Japan |
266 OC (189 M, 77 F; 20-89 y)
36527 CS (9858 M, 26669 F; 20-89 y)
|
Milk |
Age, gender, tobacco, alcohol, food groups |
7 |
|
Levi, 1998
10
|
Switzerland |
156 OC (126 M, 30 F; 26-72 y)
284 CS (246 M, 38 F; 23-74 y)
|
Milk |
Age, gender, food groups |
7 |
|
Franceschi, 1999
11
|
Italy |
271 OC (219 M, 52 F; 22-77 y)
1491 CS (1008 M, 483 F; 20-78 y)
|
Milk, cheese, butter |
Age, gender, food groups |
7 |
|
Morse, 2000
12
|
USA |
87 OC (51 M, 36 F; 20-79 y)
87 CS (51 M, 36 F; 20-79 y)
|
Milk |
Age, gender, food groups |
6 |
|
Garrote, 2001
13
|
Cuba |
200 OC (143 M, 57 F; 28-91 y)
200 CS (136 M, 64 F; 25-88 y)
|
Milk, cheese, |
Age, gender, ethnic group, education, occupation, tobacco, alcohol, food groups |
7 |
|
Petridou, 2002
14
|
Greece |
106 OC (65 M, 41 F; na)
106 CS (65 M, 41 F; na)
|
Milk |
Age, gender, education, tobacco, alcohol, BMI, food groups |
7 |
|
Lissowska, 2003
15
|
Poland |
122 OC (78 M, 44 F; na)
124 CS (72 M, 52 F; na)
|
Milk, cheese, yogurt |
Age, gender, tobacco, alcohol, genderual habits, food groups |
8 |
|
Rajkumar, 2003
16
|
India |
591 OC (309 M, 282 F; 18-87 y)
582 CS (292 M, 290 F; 18-80 y)
|
Milk, cheese, yogurt |
Age, gender, education, tobacco, alcohol, BMI, food groups |
7 |
|
Sánchez, 2003
17
|
Spain |
375 OC (304 M, 71F; 20-91 y)
375 CS (304 M, 71F; 20-87 y)
|
Milk, cheese, yogurt |
Age, gender, occupation, tobacco, alcohol, food groups |
8 |
|
Toporcov, 2004
18
|
Brazil |
70 OC (50 M, 20 F; 34-77 y)
70 CS (50 M, 20 F; 35-81 y)
|
Milk, cheese, butter |
Age, gender, food groups |
6 |
|
Gallus, 2006
19
|
Italy |
598 OC (512 M, 86 F; na)
1491 CS (1008 M, 483 F; na)
|
Milk, cheese, yogurt |
Age, gender, food groups |
7 |
|
Kreimer, 2006
20
|
USA |
1670 OC (na, na; na)
173 CS (na, na; na)
|
Milk, cheese, |
Tobacco, alcohol, BMI, food groups |
7 |
|
Marchioni, 2007
21
|
Brazil |
366 OC (310 M, 56 F; na)
469 CS (370 M, 99 F; na)
|
Milk, cheese, yogurt, butter |
Age, gender, education, tobacco, alcohol, food groups |
7 |
|
Sapkota, 2008
22
|
USA |
378 OC (331 M, 47 F; 45-74y)
916 CS (736 M, 180 F; 45-74y)
|
Milk, cheese, yogurt |
Age, gender, education, tobacco, alcohol, food groups |
8 |
|
Toporcov, 2012
23
|
Brazil |
296 OC (230 M, 66 F; na)
296 CS (230 M, 66 F; na)
|
Milk, cheese, yogurt, butter |
Age, gender, tobacco, alcohol, food groups |
7 |
|
Bravi, 2013
24
|
Switzerland |
768 OC (593 M, 175 F; 22-79 y)
2078 CS (1368 M, 710 F; 19-79 y)
|
Milk, cheese, yogurt |
Age, gender, education, BMI, food groups |
7 |
|
Chen, 2017
25
|
China |
421 OC (105 M, 316 F; 20-91 y)
1398 CS (402 M, 996 F; 20-89 y)
|
Milk |
Age, gender, education, BMI, food groups |
7 |
|
Chen, 2017
26
|
China |
930 OC (588 M, 342 F; 20-80 y)
2667 CS (1689 M, 978 F; 20-80 y)
|
Milk |
Age, gender, education, tobacco, alcohol, BMI, food groups |
7 |
OC: oral cancer patients; CS: controls without cancer; M: male; F: female; y: age range in years; na: data not available; BMI: body mass index; NOS: Newcastle-Ottawa scale.
Milk
Twenty-one studies
6-26
examined the possible influence of milk intake on oral cancer risk (Figure 2). Regular milk consumption reduced by 27% the oral cancer risk by with a highly statistically significant association (OR = 0.73; 95% CI: 0.67 to 0.78; P < 0.001).
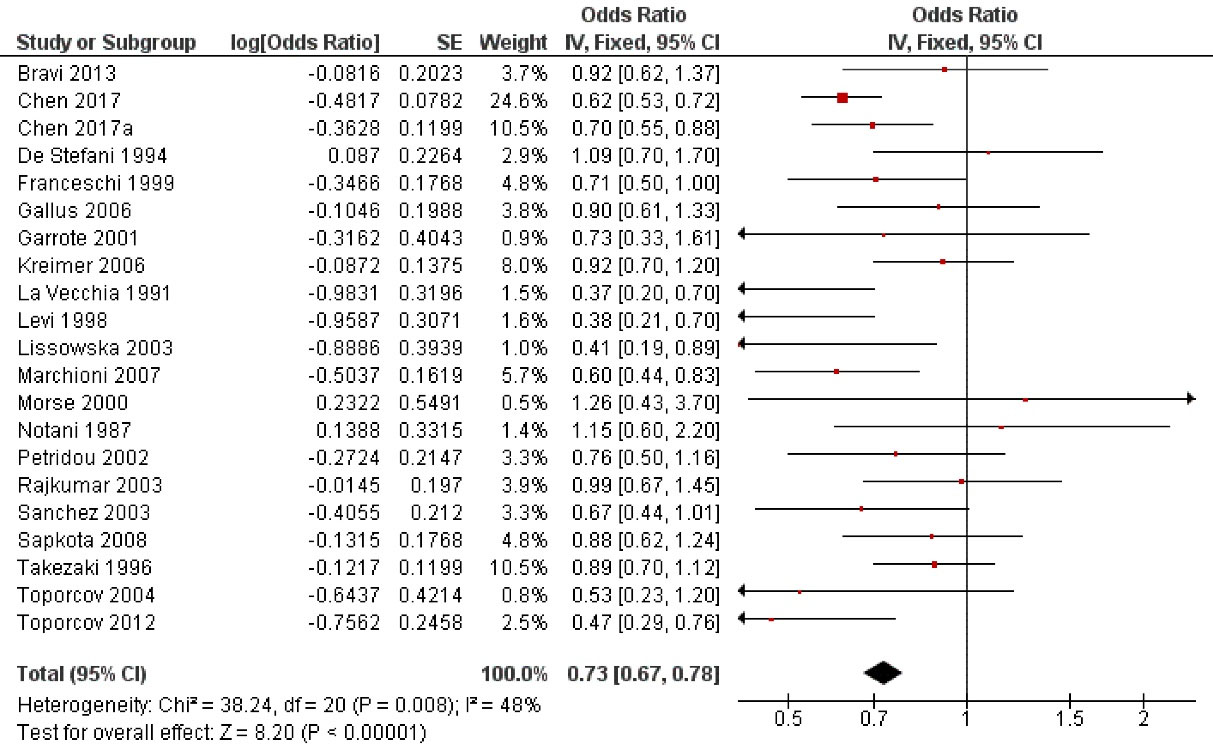
Figure 2.
Study data and forest plot graph for the influence of milk intake on oral cancer risk. SE: standard error
.
Study data and forest plot graph for the influence of milk intake on oral cancer risk. SE: standard error
Cheese
Fourteen studies
7,10,11,13,15-24
analyzed the possible influence of cheese consumption on the probability of oral cancer (Figure 3), finding that cheese decreased by 21% oral cancer risk. After statistical analysis, a highly significant relationship was found (OR = 0.79; 95% CI: 0.67 to 0.94; P < 0.001).
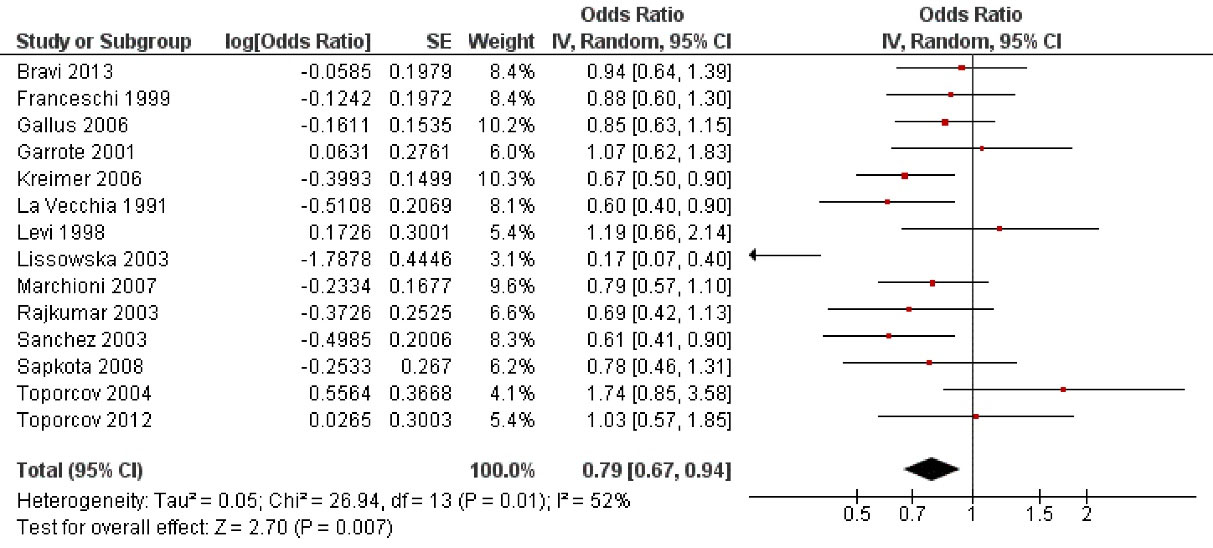
Figure 3.
Study data and forest plot graph for the influence of cheese consumption on oral cancer risk. SE: standard error
.
Study data and forest plot graph for the influence of cheese consumption on oral cancer risk. SE: standard error
Other dairy products (yogurt, butter)
Figure 4 shows the evaluation of other dairy products such as yogurt or butter. Eight studies
13,15-17,19,21-23
evaluated yogurt consumption (Figure 4A), confirming that it reduced by 25% the probability of oral cancer. Statistical analysis confirmed highly significant differences (OR = 0.75; 95% CI: 0.65 to 0.86; P < 0.001). Five studies
7,11,18,21,23
focused on butter consumption as a possible oral cancer risk factor (Figure 4B). Although butter consumption appears to reduce the oral cancer risk, the results were not statistically significant (OR = 0.75; 95% CI: 0.50 to 1.12; P = 0.16).
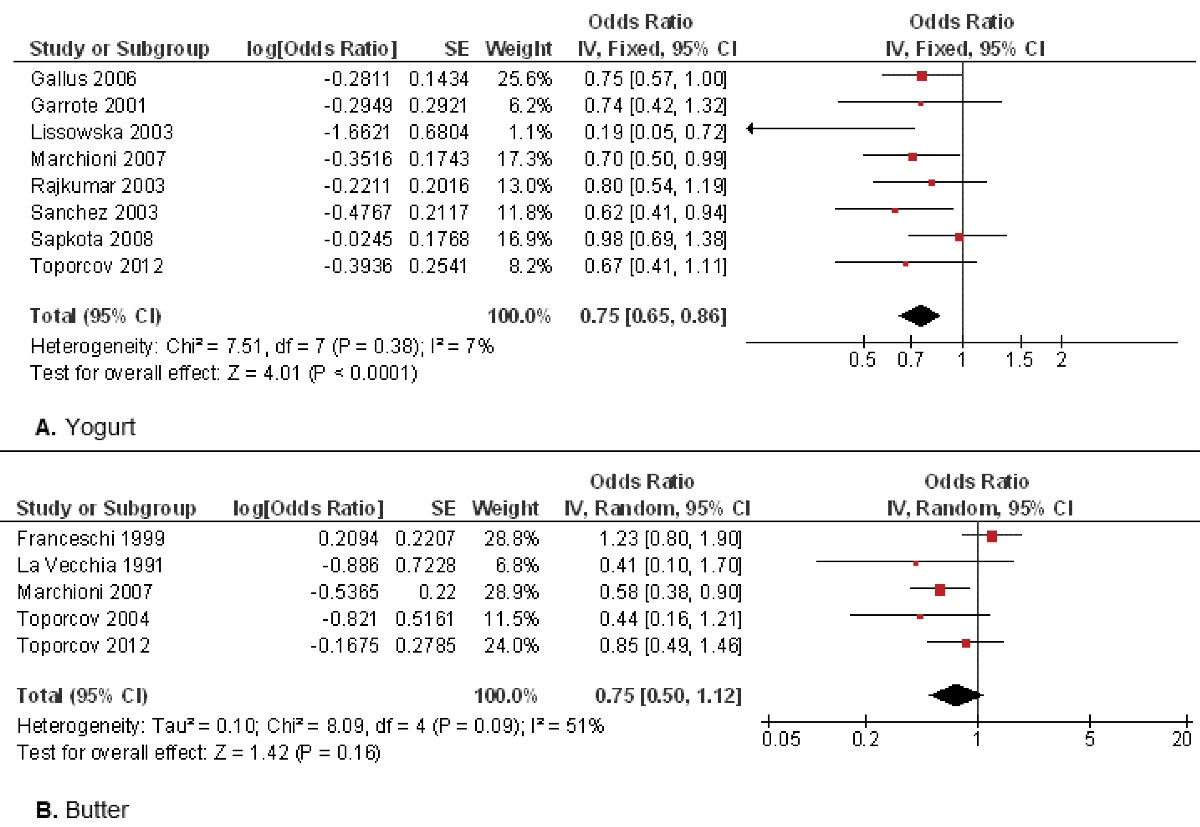
Figure 4.
Study data and forest plot graphs for the influence of yogurt consumption (A) or butter consumption (B) on oral cancer risk. SE: standard error
.
Study data and forest plot graphs for the influence of yogurt consumption (A) or butter consumption (B) on oral cancer risk. SE: standard error
Discussion
Data from 21 studies about the influence of dairy product intake (milk, cheese, yogurt, butter) on oral cancer risk were included in the present meta-analysis.
The role of regular dairy products intake on cancer risk is controversial, with apparently conflicting results depending on the location of cancer. Regarding oral cancer, some studies maintain that dairy products induce an increase in oral cancer risk, while others affirm that dairy consumption has a protective effect.
27
Many studies have confirmed the relationship between varied food components and oral cancer. The intake of red meat and dairy products has been linked to higher levels of saturated fats. The frequent intake of saturated fats from dairy products has been positively associated with higher oral cancer risk, especially in people that consume significant amounts of cakes, cheese, or ice creambars.
28
Although some studies have related dairy products to head and neck neoplasms, the real influence of these foods on the genetic transcription factors expression in oral cancers has not been established so far.
29
Using dairy products to improve oral health may have several additional health effects. Probiotic dairy products may inhibit Candida growth in the oral environment. It is thought that dairy products may change the saliva composition, such as the salivary immunoglobulins and mucins. Probiotic dairy products may be a promising choice to improve oral health, including the reduction of Candida superinfection in oral cancers.
30
In this study, regular milk consumption reduced by 27% the oral cancer risk with a highly statistically significant relationship (P < 0.001). Of the twenty-one studies that analyzed the role of milk consumption on oral cancer risk, eighteen
7,9,10,11,13-26
agreed that milk had a protective effect on cancer risk; while the remaining three
6,8,12
did not observe it. A dietary score to assess the influence of the consumption of different foods (vegetables, seafood, milk, and other dairy products) on oral cancer risk has been proposed. People who ate dairy products regularly not only had lower rates of oral cancer than those who did not but the greater the amount of dairy intake, the more this risk decreased.
26
In contrast, a study conducted in the United States found the opposite, showing that dairy product consumption was associated with the development of epithelial dysplasia, which is associated with increased oral cancer risk.
12
In the present study, cheese consumption reduced by 21% oral cancer with a highly statistically significant association (P < 0.001). Of the fourteen studies that investigated cheese consumption, ten of them
7,11,15-17,19-22,24
confirmed this lower risk of cancer-related to this food, compared to the four studies
10,13,18,23
who disagreed and did not observe this risk reduction. Studies carried out on cheese consumption and oral cancer risk showed inconsistent results, some establishing a positive association and others an inverse association. Cheese appears to have a protective effect against oral cancer, due to its high content of conjugated linoleic acid, which has immunostimulatory and anticancer properties.
22
Others do not observe this protective effect of cheese, associating its high consumption with a significant caloric intake. The intake of foods rich in calories such as cheese, other dairy products, bread, potatoes, eggs, or alcoholic beverages could explain the apparent potentiating effect of oral cancer.
18
However, moderate cheese consumption has a marked protective effect on oral cancer risk.
15
In this meta-analysis, yogurt intake reduced by 25% the oral cancer risk with highly statistically significant differences (P < 0.001). All the studies
13,15-17,19,21-23
that analyzed this food corroborated the protective effect of yogurt consumption on oral cancer. The population of Western countries has a low consumption of milk and yogurt, but considerably high consumption of cheese. The consumption of dairy products, including yogurt, does not show a consistent association with upper respiratory tract cancers, with conflicting results regarding its true influence on them. However, in the case of oral cancer, yogurt was the dairy derivative with the most favorable effect on oral cancer risk.
19
A study conducted in Brazil confirms the protective effect of consuming yogurt and other dairy products on the risk of oral cancer. Regular consumers of these dairy products, in adequate amounts, benefited from this protective effect, decreasing the incidence of oral cancer.
21
However, this effect was reversed when the intake of these foods occurred in large amounts, probably due to the large increase in calories ingested.
22
In the present study, butter consumption seemed to decrease oral cancer risk, although the results were not statistically significant (P = 0.16). Of the five studies that evaluated butter, four of them
18,20,21,23
found this protective effect of butter on oral cancer; while a single study
11
found an increased oral cancer risk associated with butter consumption. The true influence of butter intake on oral cancer risk is not well established and the results are controversially requiring further investigation.
23
However, butter consumption is a source of vitamin A and carotenoids, micronutrients with a protective effect against potentially malignant lesions and various cancers, including oral cancer. This effect is enhanced when it is consumed unprocessed as a complement to other foods.
18
A multicenter study carried out in several countries on the role of dietary habits in oral cancer risk revealed that the consumption of processed meats, butter, and alcoholic beverages were the most relevant risk factors for the appearance of oral cancer. On the contrary, the consumption of fish, raw vegetables, and oil were the most important protective factors, significantly reducing the oral cancer risk occurrence.
11
Limitations of the study
This study presents some limitations. Could not distinguish between different types of milk (whole, part-skim, skim, lactose-free, etc.) and their effect on oral cancer risk. Nor has it been possible to assess the amount of these dairy products consumed and their real influence on oral cancer risk. Finally, although the observed heterogeneity in some comparisons was not very high, the results must be interpreted with caution. New cohort studies with longer follow-up times are required to further explore the association between dairy product consumption and oral cancer risk.
Conclusion
In this meta-analysis, all dairy products significantly reduced oral cancer risk, except butter (P = 0.16). Milk intake reduced oral cancer risk by 27%, yogurt consumption by 25%, and cheese consumption by 21%.
Competing Interests
None.
Ethical Approval
Not applicable.
Funding
None.
References
- Sarode G, Maniyar N, Sarode SC, Jafer M, Patil S, Awan KH. Epidemiologic aspects of oral cancer. Dis Mon 2020; 66(12):100988. doi: 10.1016/j.disamonth.2020.100988 [Crossref] [ Google Scholar]
- Nilsson LM, Winkvist A, Esberg A, Jansson JH, Wennberg P, van Guelpen B. Dairy products and cancer risk in a Northern Sweden population. Nutr Cancer 2020; 72(3):409-20. doi: 10.1080/01635581.2019.1637441 [Crossref] [ Google Scholar]
- Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015; 101(1):87-117. doi: 10.3945/ajcn.113.067157 [Crossref] [ Google Scholar]
- Yuan J, Li W, Sun W, Deng S. Milk and dairy products consumption and the risk of oral or oropharyngeal cancer: a meta-analysis. Biosci Rep 2019; 39(12):BSR20193526. doi: 10.1042/bsr20193526 [Crossref] [ Google Scholar]
-
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, Canada: The Ottawa Hospital; 2000. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Notani PN, Jayant K. Role of diet in upper aerodigestive tract cancers. Nutr Cancer 1987; 10(1-2):103-13. doi: 10.1080/01635588709513945 [Crossref] [ Google Scholar]
- La Vecchia C, Negri E, D’Avanzo B, Boyle P, Franceschi S. Dietary indicators of oral and pharyngeal cancer. Int J Epidemiol 1991; 20(1):39-44. doi: 10.1093/ije/20.1.39 [Crossref] [ Google Scholar]
- De Stefani E, Oreggia F, Ronco A, Fierro L, Rivero S. Salted meat consumption as a risk factor for cancer of the oral cavity and pharynx: a case-control study from Uruguay. Cancer Epidemiol Biomarkers Prev 1994; 3(5):381-5. [ Google Scholar]
- Takezaki T, Hirose K, Inoue M, Hamajima N, Kuroishi T, Nakamura S. Tobacco, alcohol and dietary factors associated with the risk of oral cancer among Japanese. Jpn J Cancer Res 1996; 87(6):555-62. doi: 10.1111/j.1349-7006.1996.tb00259.x [Crossref] [ Google Scholar]
- Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer 1998; 77(5):705-9. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z [Crossref] [ Google Scholar]
- Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br J Cancer 1999; 80(3-4):614-20. doi: 10.1038/sj.bjc.6690400 [Crossref] [ Google Scholar]
- Morse DE, Pendrys DG, Katz RV, Holford TR, Krutchkoff DJ, Eisenberg E. Food group intake and the risk of oral epithelial dysplasia in a United States population. Cancer Causes Control 2000; 11(8):713-20. doi: 10.1023/a:1008943904085 [Crossref] [ Google Scholar]
- Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer 2001; 85(1):46-54. doi: 10.1054/bjoc.2000.1825 [Crossref] [ Google Scholar]
- Petridou E, Zavras AI, Lefatzis D, Dessypris N, Laskaris G, Dokianakis G. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer 2002; 94(11):2981-8. doi: 10.1002/cncr.10560 [Crossref] [ Google Scholar]
- Lissowska J, Pilarska A, Pilarski P, Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikolłajczak A. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev 2003; 12(1):25-33. doi: 10.1097/00008469-200302000-00005 [Crossref] [ Google Scholar]
- Rajkumar T, Sridhar H, Balaram P, Vaccarella S, Gajalakshmi V, Nandakumar A. Oral cancer in Southern India: the influence of body size, diet, infections and sexual practices. Eur J Cancer Prev 2003; 12(2):135-43. doi: 10.1097/00008469-200304000-00007 [Crossref] [ Google Scholar]
- Sánchez MJ, Martínez C, Nieto A, Castellsagué X, Quintana MJ, Bosch FX. Oral and oropharyngeal cancer in Spain: influence of dietary patterns. Eur J Cancer Prev 2003; 12(1):49-56. doi: 10.1097/00008469-200302000-00008 [Crossref] [ Google Scholar]
- Toporcov TN, Antunes JL, Tavares MR. Fat food habitual intake and risk of oral cancer. Oral Oncol 2004; 40(9):925-31. doi: 10.1016/j.oraloncology.2004.04.007 [Crossref] [ Google Scholar]
- Gallus S, Bravi F, Talamini R, Negri E, Montella M, Ramazzotti V. Milk, dairy products and cancer risk (Italy). Cancer Causes Control 2006; 17(4):429-37. doi: 10.1007/s10552-005-0423-2 [Crossref] [ Google Scholar]
- Kreimer AR, Randi G, Herrero R, Castellsagué X, La Vecchia C, Franceschi S. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: analysis from the IARC multinational case-control study. Int J Cancer 2006; 118(9):2293-7. doi: 10.1002/ijc.21577 [Crossref] [ Google Scholar]
- Marchioni DM, Fisberg RM, de Góis Filho JF, Kowalski LP, de Carvalho MB, Abrahão M. [Dietary factors and oral cancer: a case-control study in Greater Metropolitan São Paulo, Brazil]. Cad Saude Publica 2007; 23(3):553-64. doi: 10.1590/s0102-311x2007000300014.[Portuguese] [Crossref] [ Google Scholar]
- Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control 2008; 19(10):1161-70. doi: 10.1007/s10552-008-9183-0 [Crossref] [ Google Scholar]
- Toporcov TN, Biazevic MG, Rotundo LD, de Andrade FP, de Carvalho MB, Brasileiro RS. [Consumption of animal-derived foods and mouth and oropharyngeal cancer]. Rev Panam Salud Publica 2012; 32(3):185-91. doi: 10.1590/s1020-49892012000900003.[Portuguese] [Crossref] [ Google Scholar]
- Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer 2013; 109(11):2904-10. doi: 10.1038/bjc.2013.667 [Crossref] [ Google Scholar]
- Chen F, Yan L, Lin L, Liu F, Qiu Y, Liu F. Independent and joint effects of tea and milk consumption on oral cancer among non-smokers and non-drinkers: a case-control study in China. Oncotarget 2017; 8(30):50091-7. doi: 10.18632/oncotarget.15096 [Crossref] [ Google Scholar]
- Chen F, Yan L, Lin L, Liu F, Qiu Y, Wang J. Dietary score and the risk of oral cancer: a case-control study in southeast China. Oncotarget 2017; 8(21):34610-6. doi: 10.18632/oncotarget.16659 [Crossref] [ Google Scholar]
- Rodríguez-Molinero J, Migueláñez-Medrán BDC, Puente-Gutiérrez C, Delgado-Somolinos E, Martín Carreras-Presas C, Fernández-Farhall J. Association between oral cancer and diet: an update. Nutrients 2021; 13(4):1299. doi: 10.3390/nu13041299 [Crossref] [ Google Scholar]
- Fan Y, Qiu Y, Wang J, Chen Q, Wang S, Wang Y. Association between dietary fatty acid pattern and risk of oral cancer. Front Nutr 2022; 9:864098. doi: 10.3389/fnut.2022.864098 [Crossref] [ Google Scholar]
- Panahipour L, De Biasi M, Bokor TS, Thajer A, Haiden N, Gruber R. Milk lactoperoxidase decreases ID1 and ID3 expression in human oral squamous cell carcinoma cell lines. Sci Rep 2020; 10(1):5836. doi: 10.1038/s41598-020-62390-4 [Crossref] [ Google Scholar]
- Farias da Cruz M, Baraúna Magno M, Alves Jural L, Pimentel TC, Masterson Tavares Pereira Ferreira D, Almeida Esmerino E. Probiotics and dairy products in dentistry: a bibliometric and critical review of randomized clinical trials. Food Res Int 2022; 157:111228. doi: 10.1016/j.foodres.2022.111228 [Crossref] [ Google Scholar]