J Dent Res Dent Clin Dent Prospects. 18(2):135-142.
doi: 10.34172/joddd.40958
Original Article
Expression of IL-1β, IL-6, TNF-α, and a-MMP-8 in sites with healthy conditions and with periodontal and peri-implant diseases: A case-control study
Renzo Guarnieri Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, 1, 2 
Rodolfo Reda Conceptualization, Investigation, Writing – original draft, Writing – review & editing, 2, 3, * 
Dario Di Nardo Investigation, Validation, Visualization, Writing – review & editing, 2, 4 
Gabriele Miccoli Data curation, Formal analysis, 2 
Francesco Pagnoni Validation, Writing – review & editing, 2 
Alessio Zanza Formal analysis, Validation, Writing – review & editing, 2 
Luca Testarelli Project administration, Supervision, Validation, Writing – review & editing, 2 
Author information:
1Private Practice, Treviso, Italy
2Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, Rome, Italy
3Department of Prosthodontics and Implantology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, India
4Operative Research Unit of Dentistry, Policlinico Universitario Campus Bio-Medico Foundation, Via Alvaro del Portillo, Roma, Italy
Abstract
Background.
This study evaluated the gingival crevicular fluid (GCF) and Peri- implant crevicular fluid (PICF) concentrations of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and active metalloproteinase-8 (a-MMP-8) in sites with healthy conditions vs. sites affected by periodontitis (PER) and peri-implantitis (PIM).
Methods.
Periodontally healthy (PH) sites with PER, sites with peri-implant health (PIH), and sites with PIM were investigated intra-individually, according to the inclusion criteria of each group. Probing pocket depth (PPD), plaque index, gingival index, and the presence or absence of bleeding on probing (BoP) were evaluated. In GCF and PICF samples, IL-1β, IL-6, and TNF-α were quantified by ELISA Duoset® kit in combination with Ultramark® micro-ELISA digital reader; a-MMP8 concentration was analyzed by a chairside test (Perio/ImplantSafe®) in combination with a digital reader (ORALyzer®).
Results.
The concentrations of IL-6 and IL-1β, TNF-α, and a-MMP-8 were significantly higher in the PIM and PER sites compared to healthy sites (P<0.05). Significantly higher concentrations of IL-1β and a-MMP-8 were found in PIM vs. PER sites (P<0.05), while the concentrations of IL-6 and TNF-α did not differ between the PIM and PER groups (P>0.05).
Conclusion.
aMMP-8, IL-6, IL-1β, and TNF-α presented higher GCF/PICF concentrations in diseased periodontal and peri-implant sites. However, only the concentrations of IL-1β and a-MMP-8 were significantly higher in PIM than in PER sites.
Keywords: a-MMP-8, GCF, Gingival crevicular fluid, Interleukins, Peri-implant crevicular fluid, PICF
Copyright and License Information
©2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Periodontitis (PER) and peri-implantitis (PIM) are polymicrobial inflammatory diseases that destroy the tissues supporting the tooth/implant.1 Although the etiology of PER and PIM is currently recognized to be similar,2 a different pathogenesis, mediated by a different immune-inflammatory host response to the microbial insult, seems to be responsible for the tissue damage.3,4 In contrast to PER, PIM lesions show a differing cell profile with high numbers of B cells, osteoclasts, and neutrophils.5 PER and PIM also show a different quantitative transcript profile, suggesting the prevalence of bacterial response in PER tissues, with the prevalence of innate immune response in PIM tissues.6 Moreover, the destruction of tissues in PIM appears to be of significantly greater severity than occurs in PER.7,8
In healthy conditions, the periodontal and peri-implant tissues that are in close contact with the dental biofilm show a low-grade active immune response, which is physiological.9 The transition to a pathological condition begins with an immune-cellular activation process, which results in dysregulation in the production of inflammatory mediators such as cytokines, chemokines, prostaglandins, and proteolytic enzymes that, in turn, alter the connective tissue and bone metabolism.9-11 During the last years, an increasing interest in the assessment of some inflammatory cytokines, such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and enzymes, such as matrix metalloproteinase-8 (MMP-8) within the gingival crevicular fluid (GCF) and peri-implant crevicular fluid (PICF) has been targeted for periodontal and peri-implant disease detection and prediction to elucidate a broad overview of the pathogenesis of these diseases.12-16 IL-1β, IL-6, and TNF-α are released from the cells of the gingival epithelium, dendritic cells, connective tissue fibroblasts, macrophages, and neutrophils. They mediate the production of prostaglandin E2, leukotrienes, and platelet-activating factor and promote osteoclast activation and bone resorption. IL-1β regulates the degradation of extracellular matrix components of the plasminogen system and the collagenase activity in inflammation and wound healing. TNF-α induces fibroblast apoptosis and reduces the repair capacity of the peri-implant tissue. Furthermore, IL-1β, IL-6, and TNF-α induce tissue destruction and bone resorption by activating collagenase and through the receptor activator of nuclear factor-kappa B ligand, which stimulates osteoclast differentiation.12-16 Enzymes, such as MMP-8, are produced by neutrophils, fibroblasts, and osteoclasts, resulting in collagen degradation of connective tissue and alveolar bone.17 Although several studies on GCF/PICF IL-1β, IL-6, TNF-α, and MMP-8 levels have been performed for periodontal and peri-implant disease detection, so far, limited research has compared the levels of these biomarkers in clinical situations of periodontal/peri-implant health (PIH) and disease.18-20 Since the disease susceptibility, the immune response, and the inflammatory mediator’s profile, being individual, vary between subjects,21 the present study evaluated the intra-individualGCF/PICF concentrations of IL-1β, IL-6, TNF-α, and a-MMP-8 in sites with healthy conditions vs. sites affected by PER and PIM. The null hypothesis of this study was that there are differences in GCF/PICF IL-1β, IL-6, TNF-α, and MMP-8 levels between periodontal and peri-implant diseases. In addition, this study sought to assess the differences between healthy and diseased sites.
Methods
A clinical and radiographic investigation of dental implant therapy was performed in 2021 after approval by the Ethics Committee of the Campus Bio-Medico University of Rome. Adult subjects were selected ( > 18 years old), male or female, with generalized PER, periodontal health, PIM, or PIH. All eligible individuals were invited to participate in the study and were thoroughly informed of its nature, potential risks, and benefits. The study was conducted in accordance with the Declaration of Helsinki, and participants agreeing to participate in the study signed an informed consent form. For inclusion in the study, patients had to be > 18, systemically healthy, partially edentulous, with one or more missing teeth restored with fixed implant-supported restorations loaded for at least 12 months. Subjects with systemic diseases affecting the healing process (e.g., uncontrolled diabetes mellitus) were excluded from this study. Smoking patients, patients with liver/kidney or salivary gland dysfunction, under cancer therapy or organ transplant, pregnant or lactating women, individuals using antibiotics or immunosuppressive medication within the last three months, those needing antibiotics for infective endocarditis prophylaxis during dental procedures, those having orthodontic appliances, patients presenting oral mucosal inflammatory conditions, HIV-positive patients, and those with a history of hepatitis were excluded. Furthermore, patients whose implants had mobility or needed guided bone regeneration or sinus elevation before implant placement were also excluded. Participants selected (n = 140) had been treated with dental implants at the same office between 2010 and 2020. Thirty patients declined the invitation, bringing the total number of included patients to 112 (80%). In addition, the mean implant installation time was five years (range: 5–10 years).
Each site categorized with PER, after conventional periodontal treatment of scaling and root planing, had to present probing pocket depth (PPD) ≥ 4 mm, bleeding on probing (BoP), and radiographic marginal bone loss ≥ 3 mm (Stage III to IV). Each site categorized as a healthy peri-implant site (the PIH group) had to present the absence of peri-implant signs of soft tissue inflammation (redness, swelling, and BoP) and the absence of further additional bone loss following initial healing. Each site categorized with PIM had to present BoP and/or suppuration, with a probing depth (PD) > 4 mm, and radiographic bone loss > 3 mm, with at least 50% of peri-implant bone remaining (otherwise, the implant was considered lost). Baseline bone level measurements on radiographs from implant surgery were reduced by 1 mm to compensate for the anticipated initial bone remodeling. If the individual had more than one implant affected by PIM and more than one tooth affected by PER only, one implant and one tooth were evaluated.
The PD (6 sites per tooth), plaque index, gingival index, and modified sulcular bleeding index were employed to assess the periodontal clinical status. The clinical status of peri-implant tissues was evaluated by assessing the PD (six sites per implant) and corresponding indices for implants, including a modified plaque index (mPI),21 simplified gingival index (sGI),22 and a modified bleeding on probing index (mBOPI).21 To avoid the risk of IL-1β, IL-6, TNF-α, and aMMP-8 fluctuation due to mechanical irritation, the clinical examination was performed a week before PICF and GCF sampling.
The sampling site was prepared for GCF/PICF analysis by removing excess saliva with a short, gentle blast of air. Using tweezers, a sterile PICF collection strip was placed apically as deeply as possible into the sulcus at the sampling site. Following the manufacturer’s instructions, the a-MMP-8 levels were determined by the a-MMP-8 PoC/chairside mouthrinse test (PerioSafe®) in combination with a digital reader (ORALyzer®), whereas IL-1β, IL-6, and TNF-α were quantified by ELISA-Duoset® kit in combination with Ultramark® micro-ELISA digital reader.
A computer-assisted measurement automatically provided by a software program (VixWin Platinum, Gendex) was used for radiographic measurements of digital radiographs taken with the same radiologic device. The distance between the prosthetic connector and the peri-implant alveolar bone crest was measured to obtain vertical bone loss. These measurements were made by a single examiner who was previously trained and were used to complement the diagnosis of PIM.
Examiner calibration
Calibration exercises for clinical parameters and IL-1β, IL-6, TNF-α, and aMMP-8 estimation were performed in ten sites before the actual study. Clinical recordings were done twice by a single examination within one month. The order of patients was masked and changed between the examinations. The examiner received training before the study regarding the use of each index employed for periodontal and peri-implant examinations. PD was measured using a pressure-sensitive probe (Florida Probe, Gainesville, FL, USA), and the estimation was judged to be reproducible if the agreement within ± 1 mm between repeated measurements was at least 90%. The intra-examiner agreement between the two measurements was found to be 91%. A different examiner, who was blinded to the clinical records of the patients, carried out the fluid sampling.
Statistical analysis
IL-1β, IL-6, TNF-α, and aMMP-8 were considered the primary outcome. Based on the IL-1β, IL-6, TNF-α, and a-MMP-8 differences between the groups, the sample size was determined according to previously published data. The following parameters were used for the sample size calculation: a minimum expected difference between means of 0.5, standard deviations on the difference between means of 0.7, an effect size of 0.71, a beta error of 10%, and one-tailed alpha error of 5%, with an 80% power. This resulted in a required sample size of 56 patients. However, based on the anticipated individual variations in IL-1β, IL-6, TNF-α, and a-MMP-8 responses and the specific study design accounting for potential losses and refusals, the sample size was doubled. These calculations thus estimated a minimum of 112 patients. Data were analyzed using IBM SPSS Statistics (Version 23.0 for Windows; IBM Corp). The Wilcoxon signed-rank test was used for intra-individual pairwise comparisons of IL-1β, IL-6, TNF-α, and aMMP-8 levels and PPD/BoP; Spearman’s rank correlation test was used to determine intra-individual correlations between sites regarding IL-1β, IL-6, TNF-α, and aMMP-8 levels. For all parameters, P values < 0.05 were considered statistically significant.
Results
A total of 295 GCF/PICF samples (112 in periodontally healthy [PH] sites, 57 in PER sites, 94 in PIH sites, and 31 in PIM sites) collected from 112 patients were examined. Table 1 presents the mean periodontal clinical parameters ( ± SD) of the study population.
Table 1.
Mean periodontal clinical parameters ( ± SD) of the study population
|
Site
|
PI/mPI
|
GI/mGI
|
PD (mm)
|
% BoP
|
| PH (n = 112) |
0.84 ± 0.73 |
1.08 ± 0.18 |
2.2 ± 0.3 |
14.7 ± 5.5 |
| PER (n = 57) |
1.48 ± 0.84(*) |
1.5 ± 0.33 |
3.74 ± 0.7(**) |
72.9 ± 16.1(***) |
| PIH (n = 94) |
1.05 ± 0.13 |
1.04 ± 0.14 |
2.6 ± 0.8 |
12.1 ± 5.1 |
| PI (n = 32) |
1.25 ± 0.39 |
1.37 ± 0.41(†) |
5.5 ± 1.2(††^) |
82.3 ± 17.3(†††^^) |
PH, periodontally healthy; PER, periodontitis; PIH, peri-implant health; PI, Peri-implantitis.
Wilcoxon signed-rank test: significant difference from PH, (*)P = 0.024; (**)P = 0.037; (***)P = 0.028
Significant difference from PIH, (†)P = 0.041, (††)P = 0.014, (†††)P = 0.033,(^) significant difference from PER, (^)P = 0.028, (^^)P = 0.011.
The mean age was 59.8 ± 12.3 years, and the majority (64) were female. Seventy-seven subjects (60%) had a diagnosis of PER and 22 (20%) PIM. The mean number of dental implants was 2.7 (range: 1–5), and the mean number of residual teeth was 22.4 (range: 12–32). The mean number of sites with PPD ≥ 4 was 8.4 (range: 4–42).
The mean PI/mPI, PD, and percentage of BoP values were significantly (P < 0.017, P < 0.032, and P < 0.042, respectively) higher in PER/PIM sites than in healthy sites. Compared to PER, PIM sites showed significantly higher values of PD and BoP (P < 0.035 and P < 0.042, respectively). Table 2 presents the GCF/PICF levels of all biomarkers studied.
Table 2.
The means of IL-6, IL-1β, TNF-α, and aMMP-8 levels in GCF/PICF samples and intra-individual pairwise comparisons between groups
|
|
PH
|
PER
|
PIH
|
PI
|
| IL-1β (pg/mL) |
24.12 ± 7.2 |
41.19 ± 15.78 (*) |
22.73 ± 3.1 |
59.32 ± 10.04 (†) (^) |
| IL-6 (pg/mL) |
4.22 ± 3.4 |
7.71 ± 56.1 (**) |
4.38 ± 4.3 |
8.63 ± 5.8 (††) |
| TNF-α (pg/mL) |
14.14 ± 1.67 |
51.08 ± 12.04 (***) |
15.19 ± 2.93 |
47.88 ± 11.39 (†††) |
| aMMP-8 (ng/mL) |
11.58 ± 3.1 |
17.51 ± 9.3 (****) |
12.42 ± 2.9 |
29.8 ± 10.6 (††††) (^^) |
PH, periodontally healthy; PER, periodontitis; PIH, peri-implant health; PI, Peri-implantitis.
Wilcoxon signed-rank test: significant difference from PH (*) P = 0.043, (**) P = 0.022; (***) P = 0.031, (****) P = 0.027; significant difference from PIH (†) P = 0.036, (††) P = 0.028, (†††) P = 0.039, (††††) P = 0.055; significant difference from PER (^) P = 0.043, (^^) P = 0.022.
The concentration of a-MMP-8, IL-6, IL-1β, and TNF-α were significantly higher in the PIM and PER sites compared to the healthy sites (Figure 1). Significantly higher concentrations of IL-1β and a-MMP-8 were found in PIM vs. PER sites, while the concentrations of IL-6 and TNF-α did not differ between the PIM and PER groups.
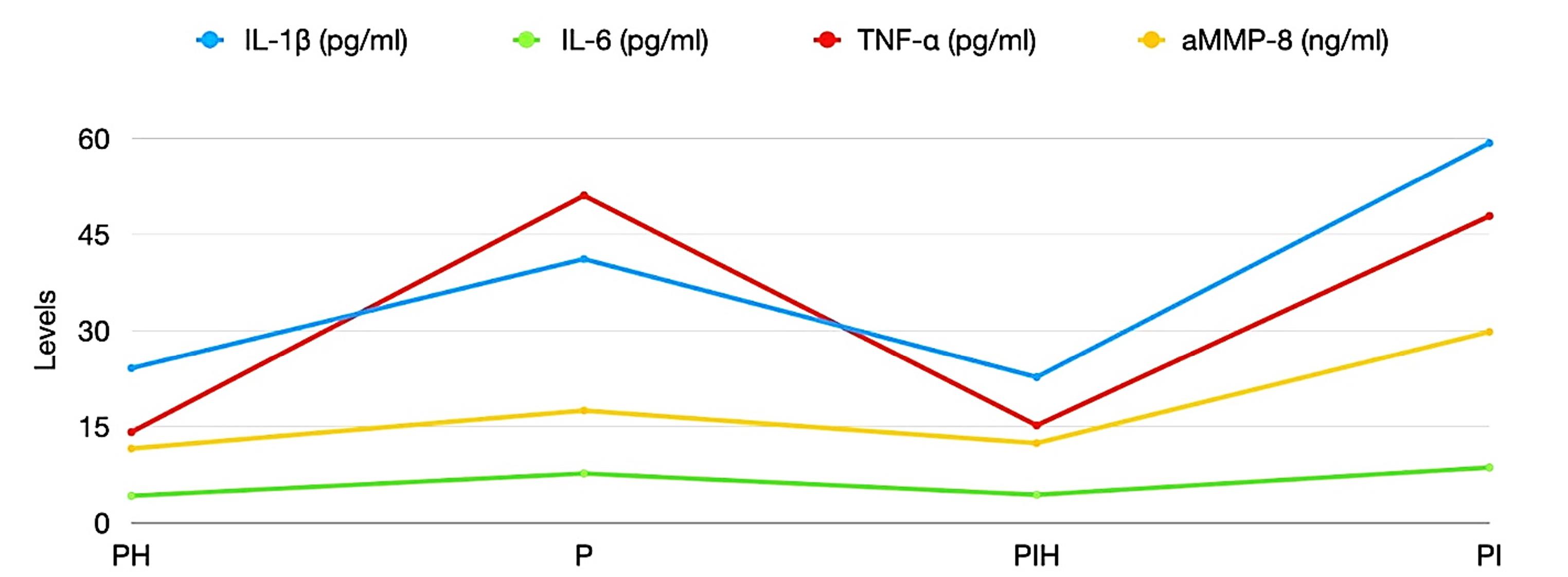
Figure 1.
Means of the intra-individual levels of the investigated biomarkers. PH, periodontally healthy; PER, periodontitis; PIH, peri-implant health; PI, Peri-implantitis
.
Means of the intra-individual levels of the investigated biomarkers. PH, periodontally healthy; PER, periodontitis; PIH, peri-implant health; PI, Peri-implantitis
Discussion
Several studies have attempted to clarify the role of cytokines in periodontal and peri-implant tissues’ immuno-inflammatory response; however, the literature presents scarce comparative data regarding the concentration of these biomarkers in PER and PIM and their role in the progression of these diseases.9,18,19,23 The present study evaluated differences in CGF/PICF levels of IL-1β, IL-6, TNF-α, and aMMP-8 in healthy, PER, and PIM sites. The null hypothesis was to verify differences in levels of these biomarkers between periodontal and peri-implant diseases. The results indicated that, compared to the healthy sites, the concentration of IL-6, IL-1β, and TNF-α were significantly higher in both PIM and PER sites. These results agree with previously published data indicating that the levels of these proinflammatory cytokines in GCF and PICF were higher than in healthy controls and that their secretion is closely related to the progression of diseases.12-15,18,19 In addition, current studies indicated that only the concentration of IL-1β was significantly higher in PIM sites compared to PER sites, while the concentrations of IL-6 and TNF-α did not differ between the PIM and PER groups.
Although some evidence suggests that IL-1β, IL-6, and TNF-α act synergistically to initiate and propagate inflammation and stimulate osteoclasts, inducing bone resorption,24,25 recent reports indicated that they have different roles in the two types of immune response, acquired and innate. IL-6 is prevalently involved in adaptive immunity and immune reactions to infection.26 Many studies have shown that IL-6 is related to infectious diseases, promoting the differentiation of B cells, the secretion of antibody proteins, and the activation of cytotoxic T cells.27-29 Involvement of IL-6 in the pathogenesis of periodontal disease is well-recognized, and IL-6 has been shown to possess potential characteristics to be used as a valid, sensitive, and specific biomarker for periodontal diseases.30 Literature data on IL-6 expression in PIM are contrasting, and no conclusive evidence has been found to prove their usefulness as markers of peri-implant disease.31-36 Based on the current study, indicating that the concentrations of IL-6 did not differ between the PIM and PER groups, it is possible to hypothesize that PER and PIM present the same degree of adaptive immune reaction to infection.
IL-1β is produced essentially by macrophages, followed by neutrophilic granulocytes, monocytes, lymphocytes, and fibroblasts, and possesses a broad spectrum of inflammatory and immunologic properties playing a crucial role in the innate immune system.26 Levels of IL-1β failed to discriminate between periodontal health and disease.37 On the contrary, several investigations reported that the levels of IL-1β were positively correlated to failing dental implants at the patient and site level, indicating IL-1β as a promising candidate in differentiating PER from healthy implants.36,38,39 Two distinct signals are needed for the expression, activation, and release of active IL-1β, namely primary and secondary signals.40 Several bacterial products, among which lipopolysaccharide, work as a primary stimulus, resulting in the up-regulation of the expression of pro-IL-1β and accumulation of pro-IL-1β intracellularly in macrophages. A secondary signal induced by direct interactions of various bacterial species or bacterial components (such as for Salmonella typhimurium or Francisella tularensis) or cell-surface interactions (such as for Listeria monocytogenes, Staphylococcus aureus, Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis), through the activation of the inflammasome complex and caspase-1 activation is ultimately required for IL-1β secretion.41-48 Recent studies indicate that titanium wear ions/particles may also function as a secondary stimulus to activate the inflammasome in macrophages, resulting in the release of active IL-1β from cells.49 Titanium particles from dental implant surfaces have been observed in soft and hard peri-implant tissues. Although their impact on the pathogenesis of PIM and in the host osteoimmunoinflammatory response is still indefinite, some studies have related the presence of these particles to peri-implant tissue inflammatory processes.48-52 In vitro investigations indicated that mice immune cells stimulated by lipopolysaccharides in the presence of titanium ions showed increased release of IL-1β was involved in bone resorption.53 In addition, titanium particles have been shown to increase the release of IL-1β by macrophages and induce secretion of receptor activator of nuclear factor-kappa B ligand upon entering T-cells, stimulating bone resorption.54 The long-standing accumulation of oral microbial biofilm on implant surfaces combined with mechanical strain may cause titanium implant surfaces to deteriorate,55-57 inducing a pathogenic cycle from the interaction between titanium and bacterial plaque that leads to definitive alterations on the titanium surface. In contrast, bacterial attachment and growth occur on the corroded surface. The pathogenic cycle may lead to definitive alterations on the titanium surface, whereas bacterial attachment and growth occur on the corroded surface. According to the results of the current study, it can be assumed that the higher IL-1β levels found in PIM sites, compared to PER sites, might depend on the presence of ions/particles released from dental implants, which contribute to the inflammatory reactions by stimulating inflammasome activation and IL-1β secretion in macrophages, which was substantially enhanced in the presence of microbial stimuli. However, further studies with histochemical and immunohistochemical analyses are needed to confirm this hypothesis.
In the present study, compared to healthy tissues, the levels of TNF-α showed an increase in both diseased periodontal and peri-implant tissues. However, no significant differences in levels of this mediator have been found between PER and PIM sites. In a previous study by Jansson et al,20 the intra-individual TNF-α profile has been reported to not differ between sites diagnosed with PER and those diagnosed with PIM but differ between healthy tooth and healthy implant sites. Since little data is present in the literature, further investigations are needed to comprehend the possible differential role of TNF-α in inflammation and the progression of periodontal and peri-implant diseases.
Regarding aMMP-8 levels, the present study showed an increase in this enzyme in PIM, compared to PER, suggesting its higher involvement in the pathogenesis of PIM. MMP-8, also known as “neutrophil collagenase,” is synthesized during the myelocyte stage of the development of the neutrophils, stored in the specific or secondary granules and released first upon activation of the cells by reactive oxygen species, tissue and plasma proteinases, or opportunistic microbial proteinases (alone or in concert).58 Once activated, the catalytically competent MMP, such as active MMP-8 (aMMP-8), acts as a potential initiator of interstitial collagenolysis at inflammatory sites. In an experimental study comparing ligature-induced PIM and PER lesions in mice, Hiyari et al59 histologically assessed soft tissue changes, including the destruction of the collagen matrix via MMP-8. Ligature-treated implants showed increased immunoreactivity of MMP-8 as compared to teeth. Authors attributed this observation to the fact that implants may present with some natural basal level of inflammation, making implants more readily prone to developing an inflammatory reaction than natural teeth.60,61 Different involvement of the metalloproteinases in the pathogenesis of PER and PIM has also been suggested by Borsani et al,62 who characterized the distribution of metalloproteinases both in the gingival epithelium and stroma in periodontal and peri-implant soft tissues. Another study indicated that fibroblasts from PIM granulation tissue showed the up-regulation of mRNA collagenase and reduced gene expression for tissue inhibitors of matrix metalloproteinases compared to cells collected from chronic PER granulation tissue.63 Therefore, it cannot be excluded that the production of collagenase may augment the destructive events in local tissue and, by a vicious circle, contribute to the further release of particles, which may partly be responsible for tissue destruction.
The evaluation of the biomarkers used in the present study allowed us to elucidate possible differences between the pathogeneses of PER and PIM. However, the results must be interpreted cautiously due to some limitations. Firstly, this study evaluated a relatively low number of subjects and biomarkers in each group. Secondly, most of the study population consisted of PER-prone individuals, which may limit the generalizability of the results to a general population. Furthermore, due to the cyclic progression of periodontal and peri-implant diseases, the biomarkers of immune-inflammatory events responsible for tissue breakdown may not always be detected with a single moment of fluid collection.
Conclusion
Within the limitations of the current study, the results indicated that the GCF/PICF levels of IL-6, IL-1β, TNF-α, and aMMP-8 differed between healthy teeth and sites diagnosed with PER and between healthy implant sites and sites diagnosed with PIM. In addition, they indicated differences in IL-1β and aMMP-8 concentrations between sites affected by PER and PIM, suggesting different pathogenic mechanisms in these diseases.
Competing Interests
The authors declare no conflicts of interest.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Università Campus Bio-Medico di Roma (protocol code Prot. PAR 30.21 (OSS) ComEt CBM-30/03/2021).
Funding
This research received no external funding.
References
- Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 2008; 35(8 Suppl):292-304. doi: 10.1111/j.1600-051X.2008.01275.x [Crossref] [ Google Scholar]
- Lang NP, Berglundh T. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011; 38 Suppl 11:178-81. doi: 10.1111/j.1600-051X.2010.01674.x [Crossref] [ Google Scholar]
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018; 45 Suppl 20:S286-91. doi: 10.1111/jcpe.12957 [Crossref] [ Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res 2003; 82(2):82-90. doi: 10.1177/154405910308200202 [Crossref] [ Google Scholar]
- Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions?. J Clin Periodontol 2011; 38 Suppl 11:188-202. doi: 10.1111/j.1600-051X.2010.01672.x [Crossref] [ Google Scholar]
- Becker ST, Beck-Broichsitter BE, Graetz C, Dörfer CE, Wiltfang J, Häsler R. Peri-implantitis versus periodontitis: functional differences indicated by transcriptome profiling. Clin Implant Dent Relat Res 2014; 16(3):401-11. doi: 10.1111/cid.12001 [Crossref] [ Google Scholar]
- Guarnieri R, Reda R, Di Nardo D, Miccoli G, Zanza A, Testarelli L. Clinical, radiographic, and biochemical evaluation of two-piece versus one-piece single implants with a laser-microgrooved collar surface after 5 years of functional loading. Clin Implant Dent Relat Res 2022; 24(5):676-82. doi: 10.1111/cid.13118 [Crossref] [ Google Scholar]
- Takamori Y, Atsuta I, Nakamura H, Sawase T, Koyano K, Hara Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin Oral Implants Res 2017; 28(2):163-70. doi: 10.1111/clr.12777 [Crossref] [ Google Scholar]
- Lasserre JF, Brecx MC, Toma S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials (Basel) 2018; 11(10):1802. doi: 10.3390/ma11101802 [Crossref] [ Google Scholar]
- Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014; 64(1):57-80. doi: 10.1111/prd.12002 [Crossref] [ Google Scholar]
- Yucel-Lindberg T, Båge T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med 2013; 15:e7. doi: 10.1017/erm.2013.8 [Crossref] [ Google Scholar]
- Kalsi AS, Moreno F, Petridis H. Biomarkers associated with periodontitis and peri-implantitis: a systematic review. J Periodontal Implant Sci 2021; 51(1):3-17. doi: 10.5051/jpis.1902840142 [Crossref] [ Google Scholar]
- Hentenaar DF, De Waal YCM, Vissink A, Van Winkelhoff AJ, Meijer HJ, Liefers SC. Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis. J Clin Periodontol 2021; 48(4):590-601. doi: 10.1111/jcpe.13423 [Crossref] [ Google Scholar]
- Guarnieri R, Reda R, Di Nardo D, Miccoli G, Zanza A, Testarelli L. In vitro direct and indirect cytotoxicity comparative analysis of one pre-hydrated versus one dried acellular porcine dermal matrix. Materials (Basel) 2022; 15(5):1937. doi: 10.3390/ma15051937 [Crossref] [ Google Scholar]
- Dursun E, Tözüm TF. Peri-implant crevicular fluid analysis, enzymes and biomarkers: a systemetic review. J Oral Maxillofac Res 2016; 7(3):e9. doi: 10.5037/jomr.2016.7309 [Crossref] [ Google Scholar]
- Ghassib I, Chen Z, Zhu J, Wang HL. Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: a systematic review and meta-analysis. Clin Implant Dent Relat Res 2019; 21(1):190-207. doi: 10.1111/cid.12694 [Crossref] [ Google Scholar]
- Arakawa H, Uehara J, Hara ES, Sonoyama W, Kimura A, Kanyama M. Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J Prosthodont Res 2012; 56(4):249-55. doi: 10.1016/j.jpor.2012.07.002 [Crossref] [ Google Scholar]
- Figueiredo LC, Bueno-Silva B, Nogueira CFP, Valadares LC, Garcia KM, da Luz Filho GC. Levels of gene expression of immunological biomarkers in peri-implant and periodontal tissues. Int J Environ Res Public Health 2020; 17(23):9100. doi: 10.3390/ijerph17239100 [Crossref] [ Google Scholar]
- Gündogar H, Uzunkaya M. The effect of periodontal and peri-implanter health on IL-1β and TNF-α levels in gingival crevicular and peri-implanter sulcus fluid: a cross-sectional study. Odovtos Int J Dent Sci 2021; 23(1):168-77. [ Google Scholar]
- Jansson L, Lundmark A, Modin C, Abadji D, Yucel-Lindberg T. Intra-individual cytokine profile in peri-implantitis and periodontitis: a cross-sectional study. Clin Oral Implants Res 2021; 32(5):559-68. doi: 10.1111/clr.13725 [Crossref] [ Google Scholar]
- Kinane DF, Preshaw PM, Loos BG. Host-response: understanding the cellular and molecular mechanisms of host-microbial interactions--consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011; 38 Suppl 11:44-8. doi: 10.1111/j.1600-051X.2010.01682.x [Crossref] [ Google Scholar]
- Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 1987; 2(4):145-51. doi: 10.1111/j.1399-302x.1987.tb00298.x [Crossref] [ Google Scholar]
- Apse P, Zarb GA, Schmitt A, Lewis DW. The longitudinal effectiveness of osseointegrated dental implants The Toronto Study: peri-implant mucosal response. Int J Periodontics Restorative Dent 1991; 11(2):94-111. [ Google Scholar]
- Gul SS, Abdulkareem AA, Sha AM, Rawlinson A. Diagnostic accuracy of oral fluids biomarker profile to determine the current and future status of periodontal and peri-implant diseases. Diagnostics (Basel) 2020; 10(10):838. doi: 10.3390/diagnostics10100838 [Crossref] [ Google Scholar]
- Guarnieri R, Reda R, Zanza A, Miccoli G, Nardo DD, Testarelli L. Can peri-implant marginal bone loss progression and a-MMP-8 be considered indicators of the subsequent onset of peri-implantitis? A 5-year study. Diagnostics (Basel) 2022; 12(11):2599. doi: 10.3390/diagnostics12112599 [Crossref] [ Google Scholar]
- Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol 2018; 9:2963. doi: 10.3389/fimmu.2018.02963 [Crossref] [ Google Scholar]
- Franza L, Carusi V, Altamura S, Caraffa A, Gallenga CE, Kritas SK. Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J Biol Regul Homeost Agents 2019; 33(5):1321-6. doi: 10.23812/Editorial [Crossref] [ Google Scholar]
- Udomsinprasert W, Jittikoon J, Sangroongruangsri S, Chaikledkaew U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-analysis. J Clin Immunol 2021; 41(1):11-22. doi: 10.1007/s10875-020-00899-z [Crossref] [ Google Scholar]
- Bonin-Jacob CM, Almeida-Lugo LZ, Puga MAM, Machado AP, Padovani CTJ, Noceti MC. IL-6 and IL-10 in the serum and exfoliated cervical cells of patients infected with high-risk human papillomavirus. PLoS One 2021; 16(3):e0248639. doi: 10.1371/journal.pone.0248639 [Crossref] [ Google Scholar]
- Costa AG, Sadahiro A, Monteiro Tarragô A, Pessoa FAC, Pires Loiola B, Malheiro A. Immune response in Mansonellaozzardi infection modulated by IL-6/IL-10 axis in Amazon region of Brazil. Cytokine 2018; 104:98-103. doi: 10.1016/j.cyto.2017.09.033 [Crossref] [ Google Scholar]
- Stadler AF, Angst PD, Arce RM, Gomes SC, Oppermann RV, Susin C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J Clin Periodontol 2016; 43(9):727-45. doi: 10.1111/jcpe.12557 [Crossref] [ Google Scholar]
- Candel-Martí ME, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali J, Peñarrocha-Diago MA. Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease An update. Med Oral Patol Oral Cir Bucal 2011; 16(4):e518-21. doi: 10.4317/medoral.16.e518 [Crossref] [ Google Scholar]
- Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M. Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol 2015; 86(5):631-45. doi: 10.1902/jop.2015.140603 [Crossref] [ Google Scholar]
- Alassy H, Parachuru P, Wolff L. Peri-implantitis diagnosis and prognosis using biomarkers in peri-implant crevicular fluid: a narrative review. Diagnostics (Basel) 2019; 9(4):214. doi: 10.3390/diagnostics9040214 [Crossref] [ Google Scholar]
- Wang HL, Garaicoa-Pazmino C, Collins A, Ong HS, Chudri R, Giannobile WV. Protein biomarkers and microbial profiles in peri-implantitis. Clin Oral Implants Res 2016; 27(9):1129-36. doi: 10.1111/clr.12708 [Crossref] [ Google Scholar]
- Fonseca FJ, Moraes Junior M, Lourenço EJ, de Moraes Teles D, Figueredo CM. Cytokines expression in saliva and peri-implant crevicular fluid of patients with peri-implant disease. Clin Oral Implants Res 2014; 25(2):e68-72. doi: 10.1111/clr.12052 [Crossref] [ Google Scholar]
- Ghassib I, Chen Z, Zhu J, Wang HL. Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: a systematic review and meta-analysis. Clin Implant Dent Relat Res 2019; 21(1):190-207. doi: 10.1111/cid.12694 [Crossref] [ Google Scholar]
- Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res 2009; 44(3):411-7. doi: 10.1111/j.1600-0765.2008.01119.x [Crossref] [ Google Scholar]
- Li JY, Wang HL. Biomarkers associated with periimplant diseases. Implant Dent 2014; 23(5):607-11. doi: 10.1097/id.0000000000000129 [Crossref] [ Google Scholar]
- Salcetti JM, Moriarty JD, Cooper LF, Smith FW, Collins JG, Socransky SS. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants 1997; 12(1):32-42. [ Google Scholar]
- Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol 2003; 15(1):26-30. doi: 10.1016/s0952-7915(02)00017-1 [Crossref] [ Google Scholar]
- Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisellatularensis is dependent on the ASC/caspase-1 axis. J Exp Med 2005; 202(8):1043-9. doi: 10.1084/jem.20050977 [Crossref] [ Google Scholar]
- Kelk P, Abd H, Claesson R, Sandström G, Sjöstedt A, Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacteractinomycetemcomitans leukotoxin. Cell Death Dis 2011; 2(3):e126. doi: 10.1038/cddis.2011.6 [Crossref] [ Google Scholar]
- Kelk P, Claesson R, Chen C, Sjöstedt A, Johansson A. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int J Med Microbiol 2008; 298(5-6):529-41. doi: 10.1016/j.ijmm.2007.06.005 [Crossref] [ Google Scholar]
- Kelk P, Johansson A, Claesson R, Hänström L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillusactinomycetemcomitans leukotoxin. Infect Immun 2003; 71(8):4448-55. doi: 10.1128/iai.71.8.4448-4455.2003 [Crossref] [ Google Scholar]
- Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol 2009; 157(3):415-22. doi: 10.1111/j.1365-2249.2009.03972.x [Crossref] [ Google Scholar]
- Belibasakis GN, Johansson A. Aggregatibacteractinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine 2012; 59(1):124-30. doi: 10.1016/j.cyto.2012.03.016 [Crossref] [ Google Scholar]
- Pettersson M, Kelk P, Belibasakis GN, Bylund D, Molin Thorén M, Johansson A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J Periodontal Res 2017; 52(1):21-32. doi: 10.1111/jre.12364 [Crossref] [ Google Scholar]
- Fretwurst T, Buzanich G, Nahles S, Woelber JP, Riesemeier H, Nelson K. Metal elements in tissue with dental peri-implantitis: a pilot study. Clin Oral Implants Res 2016; 27(9):1178-86. doi: 10.1111/clr.12718 [Crossref] [ Google Scholar]
- Wilson TG Jr, Valderrama P, Burbano M, Blansett J, Levine R, Kessler H. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol 2015; 86(1):9-15. doi: 10.1902/jop.2014.140363 [Crossref] [ Google Scholar]
- Olmedo DG, Nalli G, Verdú S, Paparella ML, Cabrini RL. Exfoliative cytology and titanium dental implants: a pilot study. J Periodontol 2013; 84(1):78-83. doi: 10.1902/jop.2012.110757 [Crossref] [ Google Scholar]
- Nishimura K, Kato T, Ito T, Oda T, Sekine H, Yoshinari M. Influence of titanium ions on cytokine levels of murine splenocytes stimulated with periodontopathic bacterial lipopolysaccharide. Int J Oral Maxillofac Implants 2014; 29(2):472-7. doi: 10.11607/jomi.3434 [Crossref] [ Google Scholar]
- Cadosch D, Sutanto M, Chan E, Mhawi A, Gautschi OP, von Katterfeld B. Titanium uptake, induction of RANK-L expression, and enhanced proliferation of human T-lymphocytes. J Orthop Res 2010; 28(3):341-7. doi: 10.1002/jor.21013 [Crossref] [ Google Scholar]
- Souza JC, Barbosa SL, Ariza EA, Henriques M, Teughels W, Ponthiaux P. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng C Mater Biol Appl 2015; 47:384-93. doi: 10.1016/j.msec.2014.11.055 [Crossref] [ Google Scholar]
- Franchi M, Bacchelli B, Martini D, Pasquale VD, Orsini E, Ottani V. Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials 2004; 25(12):2239-46. doi: 10.1016/j.biomaterials.2003.09.017 [Crossref] [ Google Scholar]
- Guarnieri R, Reda R, Di Nardo D, Pagnoni F, Zanza A, Testarelli L. Effects of maintenance implant therapy with and without periodic removal and decontamination of prosthetic components on inflammatory peri-implant parameters. Int J Periodontics Restorative Dent. 2023(7):s118-28. 10.11607/prd.6395.
- Wheelis SE, Gindri IM, Valderrama P, Wilson TG Jr, Huang J, Rodrigues DC. Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clin Oral Implants Res 2016; 27(3):329-40. doi: 10.1111/clr.12545 [Crossref] [ Google Scholar]
- Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta 2010; 1803(1):20-8. doi: 10.1016/j.bbamcr.2009.04.003 [Crossref] [ Google Scholar]
- Hiyari S, Wong RL, Yaghsezian A, Naghibi A, Tetradis S, Camargo PM. Ligature-induced peri-implantitis and periodontitis in mice. J Clin Periodontol 2018; 45(1):89-99. doi: 10.1111/jcpe.12817 [Crossref] [ Google Scholar]
- Borsani E, Salgarello S, Mensi M, Boninsegna R, Stacchiotti A, Rezzani R. Histochemical and immunohistochemical evaluation of gingival collagen and metalloproteinases in peri-implantitis. Acta Histochem 2005; 107(3):231-40. doi: 10.1016/j.acthis.2005.06.002 [Crossref] [ Google Scholar]
- Reda R, Zanza A, Bellanova V, Patil S, Bhandi S, Di Nardo D. Zinc oxide non-eugenol cement versus resinous cement on single implant restoration: a split-mouth study. J Compos Sci 2023; 7(3):128. doi: 10.3390/jcs7030128 [Crossref] [ Google Scholar]
- Reda R, Zanza A, Di Nardo D, Bellanova V, Xhajanka E, Testarelli L. Implant survival rate and prosthetic complications of OT equator retained maxillary overdenture: a cohort study. Prosthesis 2022; 4(4):730-8. doi: 10.3390/prosthesis4040057 [Crossref] [ Google Scholar]
- Botero JE, Yepes FL, Roldán N, Castrillón CA, Hincapie JP, Ochoa SP. Tooth and periodontal clinical attachment loss are associated with hyperglycemia in patients with diabetes. J Periodontol 2012; 83(10):1245-50. doi: 10.1902/jop.2012.110681 [Crossref] [ Google Scholar]