J Dent Res Dent Clin Dent Prospects. 2025;19(2):68-75.
doi: 10.34172/joddd.025.41492
Review Article
Survival and prognostic factors in mucosal melanomas of the oral cavity: A meta-analysis
Alberto Rodriguez-Archilla Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, 1, 2, * 
Pablo Valverde-Martinez Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, 1
Author information:
1Department of Stomatology, Oral Medicine Unit, Faculty of Dentistry, University of Granada, Granada, Spain
2Biohealth Research Institute (IBS), Granada, Spain
Abstract
Background.
The development of early metastases due to the high vascularization and lymphatic drainage of the oral cavity, followed by delayed diagnosis, results in a worse prognosis of oral melanomas. The present study aimed to determine the survival of oral melanoma in different periods and analyze its prognostic factors.
Methods.
A search for studies on survival and prognostic factors of oral melanoma was performed in the following databases: PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. The estimation of the pooled proportion was carried out with the generic inverse variance method, using the standard error (SE) of the proportion. For dichotomous outcomes, the estimates of the effects of the intervention were expressed as odds ratios (ORs) using the Mantel-Haenszel (M-H) method, all with 95% confidence intervals.
Results.
Thirty-eight studies that considered 3767 oral melanoma patients were included in this meta-analysis. Overall survival (OS) decreased from 58% at two years to 42% at three years and 29% at five years. Regarding prognostic factors, non-ulcerated oral melanomas with a high degree of pigmentation showed the best survival at 5 years. In contrast, oral tumor location and gender did not significantly affect oral melanoma survival.
Conclusion.
Oral melanoma has a low survival rate, with ulcerated and poorly pigmented tumors having the worst prognosis.
Keywords: Melanoma, Mouth, Prognosis, Risk factors, Survival
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Oral melanomas account for approximately 4% of all melanomas. They are mainly diagnosed between the 4th and 7th decades of life, with a peak incidence around 60 years of age, and are slightly more prevalent in males. Unlike cutaneous melanomas, the incidence of oral melanoma has remained stable in recent years.1 Its etiology remains unknown, although some risk factors, such as chronic inflammation induced by smoking or chronic mechanical irritation, have been suggested. Notably, a significant proportion of oral melanomas arise de novo from the apparently normal oral mucosa. However, approximately 30%‒40% of cases are preceded by oral pigmentations that persist for several months or even years.2 The main oral locations for melanomas are the palate and the alveolar gingival ridge. The lack of symptomatology associated with oral melanoma often results in patients delaying medical attention, which can lead to delayed diagnosis and a poor prognosis. Early and prompt diagnosis of oral melanomas is vital for the patient’s prognosis, as they exhibit more aggressive behavior and worse prognosis than melanomas with other locations in the body.3 Indeed, almost one-third of patients present with lymph node metastases at the time of diagnosis of primary oral melanoma. The early development of metastases may be attributed to the high level of vascularization and lymphatic drainage in the oral cavity.4 This study aimed to determine the survival of patients with oral melanoma in different periods and assess its prognostic factors.
Methods
All research steps (search, study selection, and data extraction) were achieved independently by both authors (ARA and PVM). Discrepancies in article selection were resolved by consensus.
The research question was: How do different prognostic factors influence the survival of patients with oral melanoma?
Search strategy
Table 1 shows the search strategies in each database using a combination of Medical Subject Headings (MeSH) and free-text terms. The inclusion criteria were as follows: a) all types of articles related to our purpose and b) articles written in any language and with no restrictions on publication date. The exclusion criteria were: a) articles with no full-text availability, b) articles with a relevant risk of bias (score ≤5 stars on the Newcastle-Ottawa methodological quality assessment scale),5 c) articles with no clinical data, and d) studies with non-usable data.
Table 1.
Search strategies for the three databases
|
Database
|
#
|
Search strategy
|
Results
|
| PubMed |
#1 |
“melanoma”[MeSH Terms] |
112,400 |
| #2 |
“mouth”[MeSH Terms] |
326,150 |
| #3 |
#1 AND #2 |
619 |
| #4 |
(“survival”[MeSH Terms] OR “prognosis”[MeSH Terms]) |
1,990,947 |
| #5 |
#3 AND #4 |
134 |
| Web of Science (WoS) |
#6 |
(“melanoma”[Topic] AND “mouth” [Topic]) |
834 |
| #7 |
(“survival” [Topic] OR “prognosis” [Topic]) |
1,244,600 |
| #8 |
#6 AND #7 |
247 |
| Scopus |
#7 |
TITLE-ABS-KEY (“melanoma” AND “mouth”) |
2,718 |
| #8 |
TITLE-ABS-KEY (“survival” OR “prognosis”) |
2,322,214 |
|
|
#9 |
#7 AND #8 |
227 |
Assessment of methodological quality
The methodological quality of the articles was screened using the Newcastle-Ottawa (NOS) methodological quality assessment scale,5 which is composed of eight items that evaluate three dimensions (selection, comparability, and exposure). Considering the score obtained, the studies are classified as high quality (≥7 stars), moderate quality (4‒6 stars), and low quality (0‒3 stars).
Data extraction
Overall survival (OS) of oral mucosal melanomas was established in three periods: 2, 3, and 5 years. Prognostic factors related to survival, such as the oral tumor location, the gender of the patients, the existence of ulceration of the lesion, or the degree of pigmentation of the neoplasm, were also evaluated.
Statistical analysis
For the meta-analysis, data were processed with RevMan 5.4 software (The Cochrane Collaboration, Copenhagen, Denmark). The proportion (P) was calculated by dividing the number of positive cases (n) by the total population (N). Estimation of the proportion was carried out with the generic inverse of variance method, using the standard error (SE) of the proportion and 95% confidence intervals (95% CI). The SE was obtained using the formula SQRT (P*(1-P)/N). For dichotomous outcomes, the odds ratio (OR) with the Mantel-Haenszel chi-squared formula (M-H) was used, both with 95% confidence intervals (95% CI). Heterogeneity was determined according to the Higgins statistic (I2). The random-effects model was applied in cases of high heterogeneity (I2>50%). A P-value below 0.05 was considered the minimum level of significance. The risk of publication bias was assessed using MedCalc Statistical Software version 23.1.7 (MedCalc Software Ltd., Ostend, Belgium). A funnel plot and Egger’s regression test were employed, with a minimum of 10 studies required for analysis. Publication bias was considered present if asymmetry was observed in the funnel plot and if Egger’s test yielded a P value<0.05.
Results
Study selection
In the initial search, 608 articles were found (134 in PubMed, 247 in WoS, and 227 in Scopus) between 1955 and 2021, with 229 duplicates, leaving 379 eligible articles. A total of 341 studies were excluded due to a) articles with no full-text availability (n=64), b) articles with a relevant risk of bias (score ≤5 stars on the NOS) (n=87), c) articles without clinical data (n=82), and d) studies with non-usable data (n=108). After applying these criteria, 38 studies were included in this meta-analysis (Figure 1).
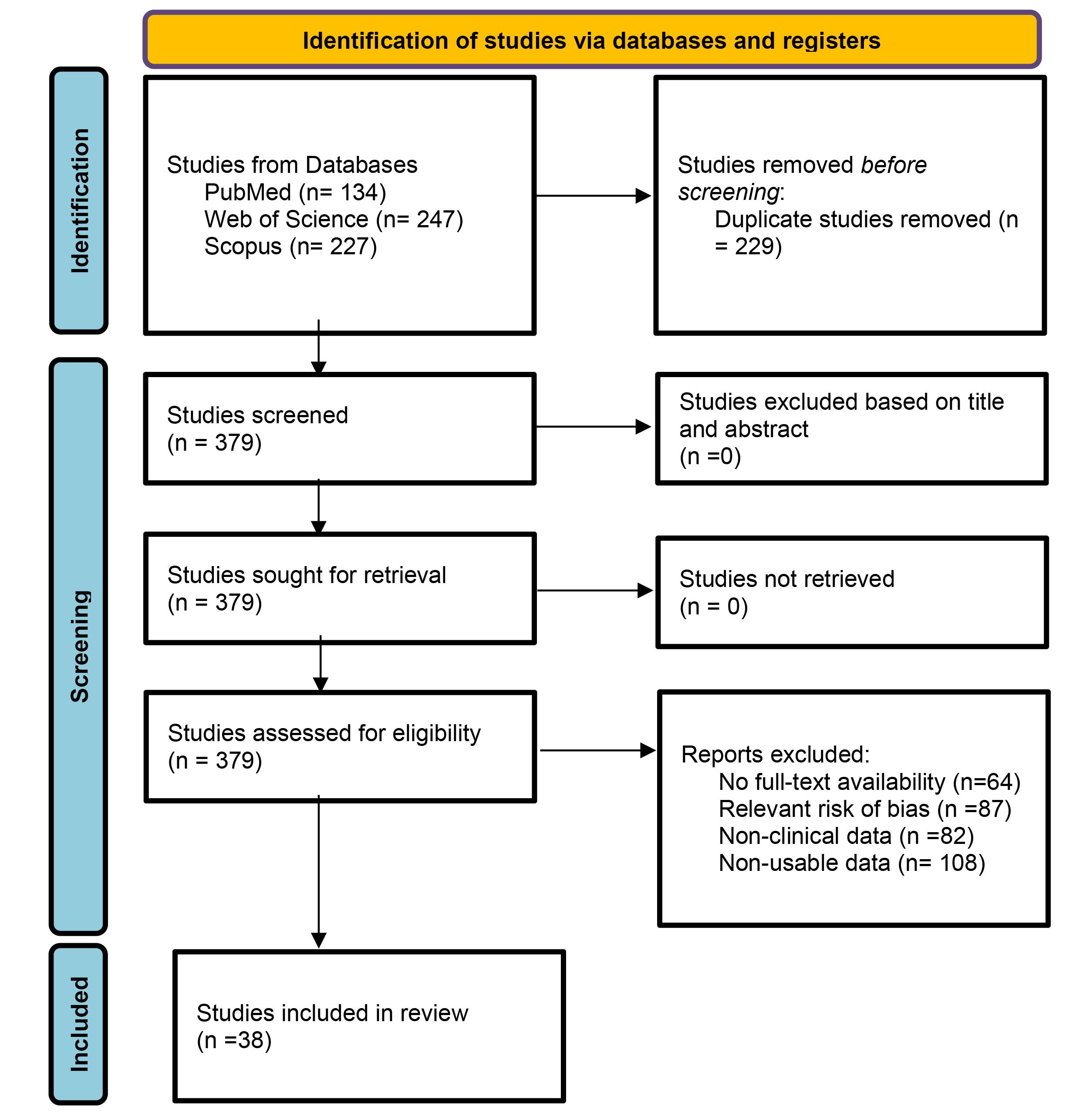
Figure 1.
Study selection flowchart
.
Study selection flowchart
Table 2 summarizes the main descriptive characteristics and the methodological quality of 38 studies6-43 included in this meta-analysis, as assessed using the Newcastle-Ottawa Scale (NOS). A total of 3767 patients with oral mucosal melanoma were analyzed, with 50.4% males and 49.6% females. The studies were conducted in the following countries: United States of America (12 studies), China (8), Brazil (3), Japan (3), Denmark (2), France (2), Germany (2), Australia (1), Canada (1), Italy (1), South Korea (1), The Netherlands (1), and Turkey (1). According to the NOS, nine studies (23.7%) received 6 stars (moderate quality), 21 studies (55.3%) received 7 stars (high quality), and 8 studies (21.0%) received 8 stars (high quality).
Table 2.
Descriptive characteristics and methodological quality evaluation of the 38 studies included in this meta-analysis
|
Study, year |
Country
|
Study populations (gender distribution, mean age)
|
Other parameters assessed
|
NOS
|
| Abraham, 20106 |
USA |
27 pat. (15M, 12F; 68y) |
Race, treatment, stage, follow-up, OS |
6 |
| Bakkal, 20157 |
Turkey |
10 pat. (6M, 4F; 66y) |
Treatment, stage, follow-up, local recurrence, regional recurrence, OS |
6 |
| Berthelsen, 19848 |
Denmark |
38 pat. (31M, 7F; 64y) |
Treatment, stage, follow-up, OS |
7 |
| Breik, 20169 |
Australia |
14 pat. (8M, 6F; 65y) |
Treatment, pathology, stage, follow-up, OS |
6 |
| Chae, 202010 |
South Korea |
74 pat. (41M, 33F; 59y) |
Treatment, bone invasion, resection margin status, stage, follow-up, OS |
8 |
| Conley, 197411 |
USA |
52 pat. (36M, 16F; 60y) |
Treatment, stage, follow-up, OS |
8 |
| Francisco, 201612 |
Brazil |
51 pat. (31M, 20F; 59y) |
Race, treatment, stage, recurrence, follow-up, OS |
8 |
| Guo, 202013 |
China |
92 pat. (40M, 52F; na) |
Treatment, pigmentation, stage, recurrence, follow-up, OS |
6 |
| Jethanamest, 201114 |
USA |
815 pat. (382M, 433F; na) |
Race, treatment, stage, follow-up, OS |
7 |
| Lawaetz, 201615 |
Denmark |
98 pat. (41M, 57F; na) |
Treatment, stage, pigmentation, ulceration, follow-up, OS |
7 |
| Lian, 201716 |
China |
706 pat. (233M, 473F; 55y) |
Treatment, stage, BRAF mutation, cKIT mutation, follow-up, OS |
7 |
| Meleti, 200817 |
The Netherlands |
14 pat. (5M, 9F; 57.9y) |
Treatment, stage, follow-up, OS |
6 |
| Moore, 195518 |
USA |
26 pat. (na, na; na) |
Race, treatment, stage, follow-up, OS |
6 |
| Moya-Plana, 201919 |
France |
45 pat. (31M, 14F; 64y) |
Treatment, stage, margins, follow-up, OS |
7 |
| Naganawa, 201620 |
Japan |
19 pat. (9M, 10F; na) |
Treatment, stage, follow-up, OS |
6 |
| Owens, 200321 |
USA |
48 pat. (39M, 9F; 55.5y) |
Treatment, stage, follow-up, OS |
7 |
| Patel, 200222 |
USA |
59 pat. (34M, 25F; 63y) |
Treatment, stage, vascular invasion, follow-up, OS |
8 |
| Perri, 201723 |
Italy |
20 pat. (14M, 6F; 54y) |
Treatment, stage, pigmentation, ulceration, follow-up, OS |
6 |
| Prasad, 200224 |
USA |
40 pat. (30M, 10F; 59.5y) |
Treatment, stage, pigmentation, ulceration, follow-up, OS |
8 |
| Prinzen, 201925 |
Germany |
50 pat. (25M, 25F; 65y) |
Treatment, stage, sentinel node biopsy, follow-up, OS |
7 |
| Rapini, 198526 |
USA |
124 pat. (78M, 46F; na) |
Treatment, stage, follow-up, OS |
7 |
| Sahovaler,202127 |
Canada |
76 pat. (33M, 43F; 66.3y) |
Treatment, stage, margins, follow-up, OS |
7 |
| Schaefer, 201728 |
Germany |
32 pat. (22M, 10F; 64.5y) |
Treatment, stage, follow-up, OS |
7 |
| Schmidt, 201729 |
USA |
326 pat. (180M, 146F; 66y) |
Treatment, stage, neck dissection, follow-up, OS |
7 |
| Shah, 197730 |
USA |
74 pat. (47M, 27F; na) |
Race, treatment, stage, follow-up, OS |
7 |
| Shuman, 201131 |
USA |
52 pat. (21M, 31F; 66y) |
Treatment, stage, margins, follow-up, OS |
7 |
| Soares, 202132 |
Brazil |
8 pat. (6M, 2F; 53.6y) |
Treatment, stage, pigmentation, biomarkers, follow-up, OS |
6 |
| Song, 201633 |
China |
62 pat. (34M, 28F; 55.4y) |
Treatment, stage, follow-up, OS |
8 |
| Song, 201734 |
China |
62 pat. (34M, 28F; 55.4y) |
Treatment, stage, BAP1 expression, follow-up, OS |
7 |
| Sun, 201235 |
China |
51 pat. (36M, 15F; na) |
Treatment, stage, pigmentation, neck dissection, follow-up, OS |
7 |
| Tanaka, 200436 |
Japan |
35 pat. (14M, 21F; 65.2y) |
Treatment, stage, follow-up, OS |
8 |
| Temam, 200537 |
France |
69 pat. (36M, 33F; na) |
Treatment, stage, follow-up, OS |
7 |
| Trodahl, 197038 |
USA |
42 pat. (36M, 6F; 49.5y) |
Treatment, stage, follow-up, OS |
7 |
| Wang, 201339 |
China |
81 pat. (50M, 31F; na) |
Treatment, stage, heparanase expression, follow-up, OS |
7 |
| Wu, 201840 |
China |
170 pat. (91M, 79F; na) |
Treatment, stage, ulceration, follow-up, OS |
8 |
| Yamada, 201741 |
Japan |
38 pat. (24M, 14F; 65.2y) |
Treatment, stage, follow-up, OS |
7 |
| Yang, 201042 |
China |
78 pat. (50M, 28F; 53.8y) |
Treatment, stage, recurrence, follow-up, OS |
7 |
| Yii, 200343 |
UK |
89 pat. (43M, 46F; 64y) |
Race, treatment, stage, follow-up, OS |
7 |
NOS: Newcastle-Ottawa methodological quality scale; pat.: patients with oral mucosal melanoma; M: male; F: female; y: mean age in years; na: data not available; OS: overall survival.
Overall survival of oral melanoma at 2 years
Five studies9,10,23,31,42 that included 216 patients with oral melanoma (Figure 2) found a pooled 2-year OS of 58% (95% CI: 44% to 71%) with high heterogeneity between studies (I2: 74%). Study variability ranged from a maximum OS of 72% (95% CI: 62% to 82%)10 to a minimum OS of 30% (95% CI: 10% to 50%).23
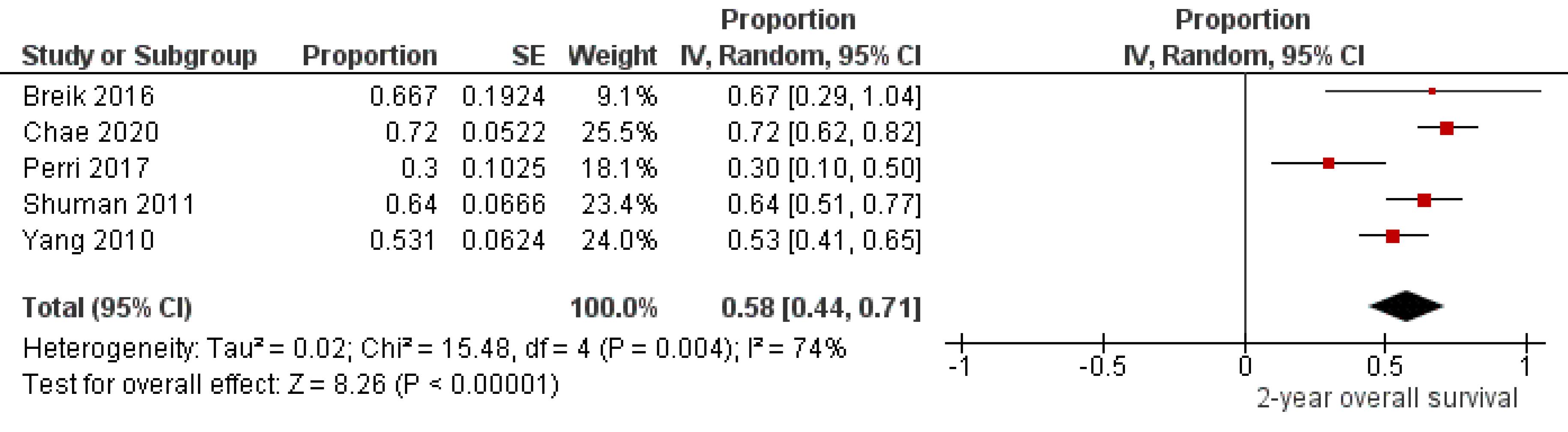
Figure 2.
Studydata and forest plot graph for 2-year overall survival of patients with oral melanoma. SE: standard error
.
Studydata and forest plot graph for 2-year overall survival of patients with oral melanoma. SE: standard error
Overall survival of oral melanoma at 3 years
Seventeen studies,6,7,13-15,19-22,26,27,29,32-35,42 including 837 oral melanoma patients (Figure 3), found a pooled 3-year OS of 42% (95% CI: 37% to 48%) with high heterogeneity between studies (I2: 81%). Study variability ranged from a maximum OS of 68% (95% CI: 47% to 89%)20 to a minimum OS of 17% (95% CI: 0% to 42%).32
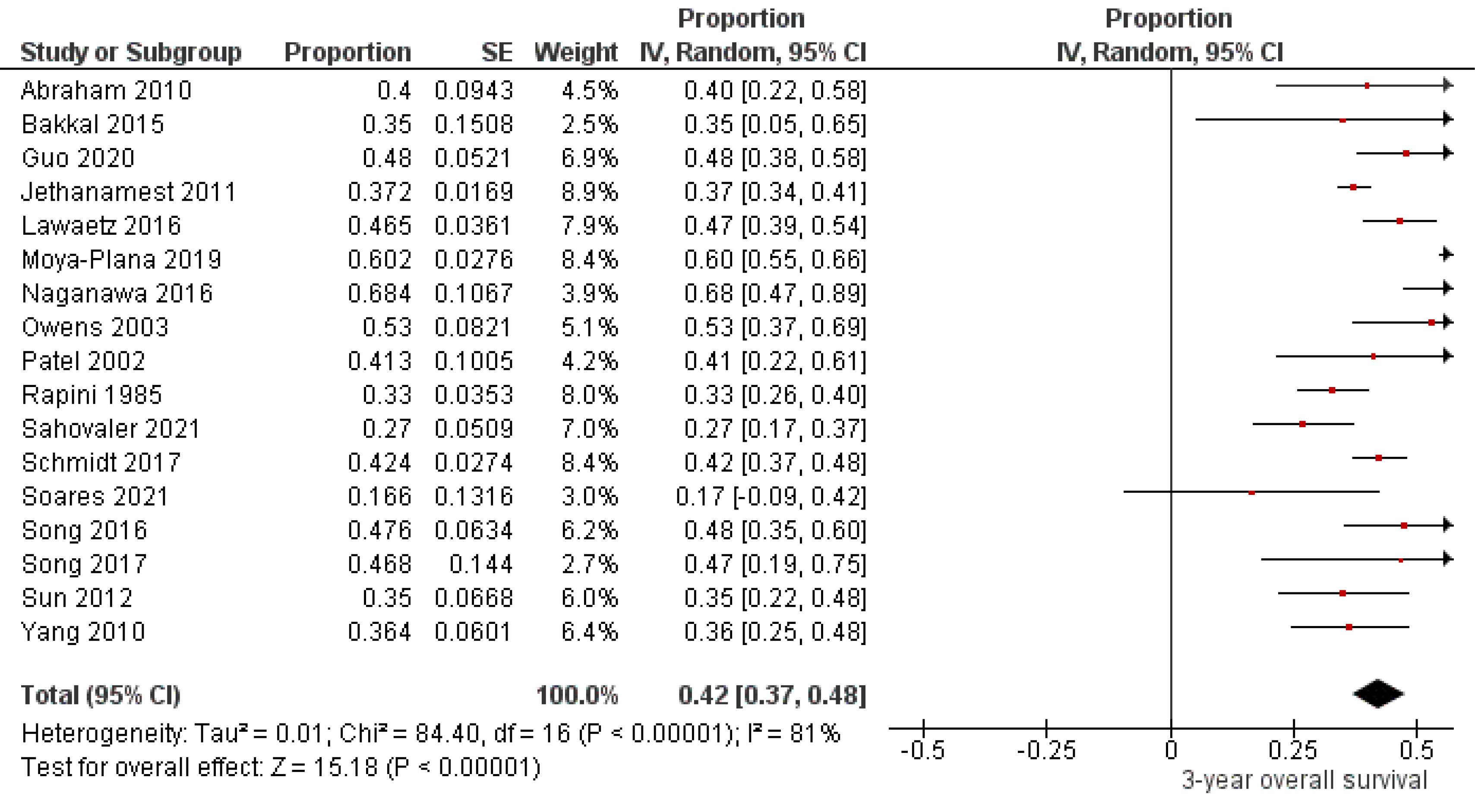
Figure 3.
Study data and forest plot graph for 3-year overall survival of patients with oral melanoma. SE: standard error
.
Study data and forest plot graph for 3-year overall survival of patients with oral melanoma. SE: standard error
Overall survival of oral melanoma at 5 years
Thirty-three studies6-11,13,14,16-22,24-26,28-39,41-43 involving 2773 patients with oral melanoma (Figure 4) found a pooled 5-year OS of 29% (95% CI: 24% to 34%) with high heterogeneity between studies (I2: 89%). Study variability ranged from a maximum OS of 57% (95% CI: 35% to 80%)20 to a minimum OS of 4% (95% CI: 0% to 12%).18
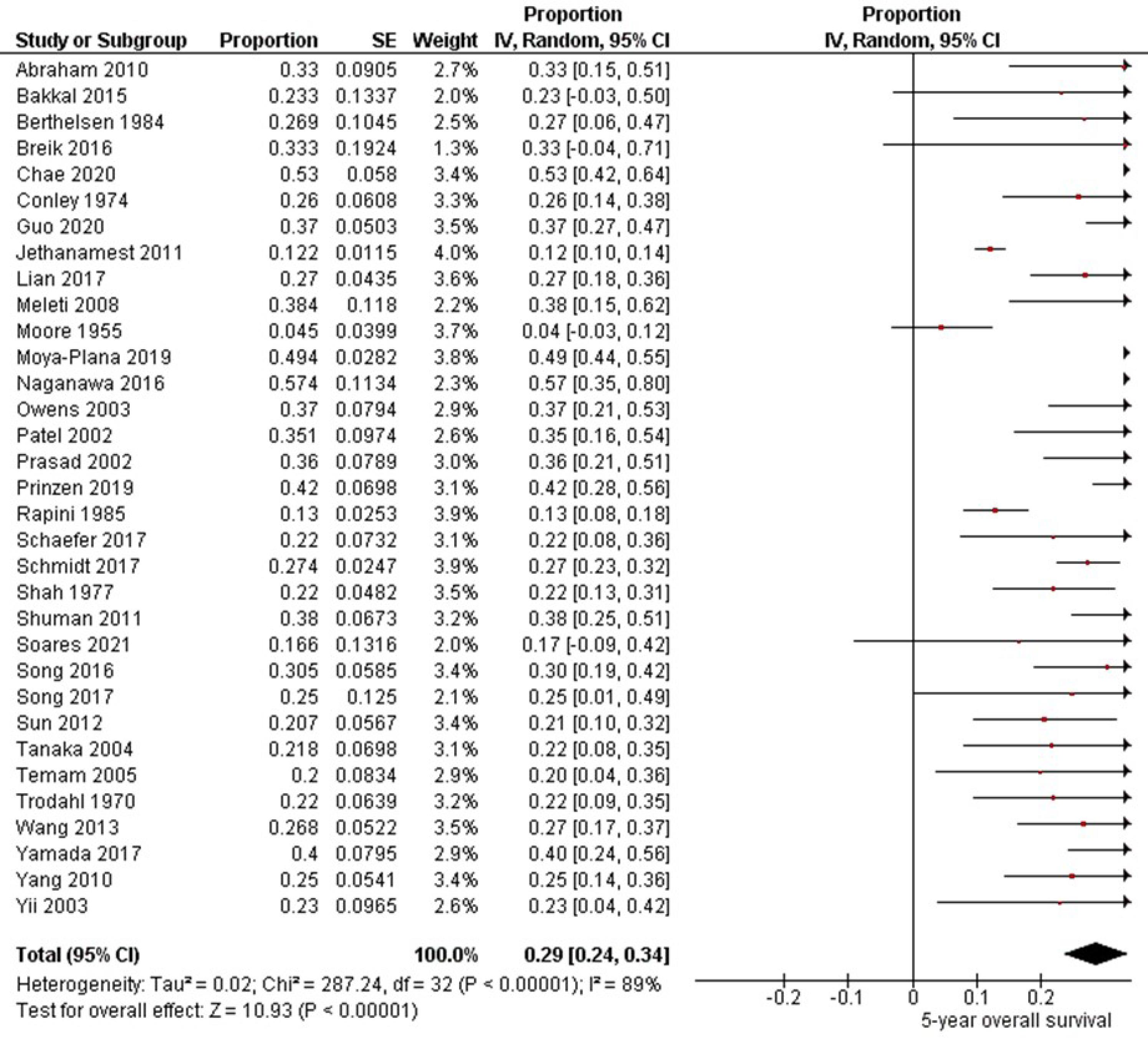
Figure 4.
Study data and forest plot graphs for 5-year overall survival of patients with oral melanoma. SE: standard error
.
Study data and forest plot graphs for 5-year overall survival of patients with oral melanoma. SE: standard error
Overall survival of oral melanoma related to other clinical parameters
Table 3 shows the possible influence of other clinical parameters (oral location of the tumor, gender, ulceration, and the degree of pigmentation) on the OS of oral mucosal melanomas.
Table 3.
Analysis of different clinical parameters related to 5-year survival of oral melanomas
|
Parameter
|
References
|
Value
|
OR
|
[95% CI]
|
I2 (%)
|
P
|
| Oral location of melanoma |
17,39,42
|
Palate |
1.49 |
[0.58 to 3.81] |
0% |
0.41 |
| Gender |
17,26,33,39,42
|
Female |
1.41 |
[0.91 to 2.18] |
0% |
0.13 |
| Ulceration |
24,25,42,43
|
No |
3.78 |
[2.24 to 6.39] |
0% |
<0.001* |
| Degree of pigmentation |
13,24-26,39
|
Highly pigmented |
2.47 |
[1.46 to 4.19] |
0% |
<0.001* |
OR: odds ratio; CI: confidence interval; I2 (%): Higgins statistic for heterogeneity (percentage); *Statistically significant.
Three studies20,39,40 evaluated the oral location of the melanoma, observing a higher probability of 5-year survival in tumors located in the palate, although without reaching statistical significance (OR=1.49, 95% CI: 0.58 to 3.81, P=0.41). Five studies20,25,35,39,40 focused on the patient’s gender. Although females exhibited a longer 5-year survival compared to males, the results were also not statistically significant (OR=1.41, 95% CI: 0.91 to 2.18, P=0.13).
Four studies12,33,34,40 compared oral ulcerated and non-ulcerated melanomas concerning 5-year survival. Patients with non-ulcerated melanomas were 3.78 times more likely to be alive at 5 years with a highly significant association (OR=3.78, 95% CI: 2.24 to 6.39, P<0.001).
Five studies13,33-35,39 examined the degree of pigmentation of melanomas. Patients with highly pigmented tumors were 2.47 times more likely to be alive at 5-year follow-up. After statistical analysis, highly significant differences were found (OR=2.47, 95% CI: 1.46 to 4.19, P<0.001).
Publication bias
Table 4 presents the assessment of publication bias based on Egger’s regression test. Publication bias was confirmed by the presence of asymmetry in the funnel plot with P<0.05 in Egger’s test for 5-year OS (P=0.0029) but not for 3-year OS (P=0.8653).
Table 4.
Analysis of publication bias according to Egger’s regression test
|
Parameter
|
n*
|
t
|
[95% CI]
|
P
value
|
| OS oral melanoma at 2 years |
5 |
NA |
NA |
NA |
| OS oral melanoma at 3 years |
17 |
0.1760 |
[-1.9971 to 2.3490] |
0.8653 |
| OS oral melanoma at 5 years |
33 |
2.3638 |
[0.8710 to 3.8566] |
0.0029 |
| Oral location of melanoma |
3 |
NA |
NA |
NA |
| Gender |
5 |
NA |
NA |
NA |
| Ulceration |
4 |
NA |
NA |
NA |
| Degree of pigmentation |
5 |
NA |
NA |
NA |
* Minimum of 10 studies to perform the analysis; OS: overall survival; n: number of studies; t: intercept; CI: confidence interval; NA: not assessable.
Discussion
The present meta-analysis on survival and prognostic factors related to oral melanoma included data from 38 studies.
In this study, the 2-year survival of oral melanomas was 58%. Of the five studies that analyzed this parameter, four9,10,31,42 reported percentages similar to the current paper, whereas one study23 found a considerably lower survival (30%). In the present study, the 3-year survival of oral melanomas reached 42%. Of the seventeen studies that evaluated this variable, thirteen6,7,13-15,21,22,26,29,33-35,42 found similar percentages, two19,20 found significantly higher percentages (>60%), and two other studies27,32 found much lower survival percentages (<30%). In this study, the 5-year survival rate for oral melanoma was 29%. Of the 35 studies examining this parameter, 18 studies6-9,11,16,22,24,28-30,32,33,36,38,39,42,43 reported a 5-year survival percentage similar to that of this study (range: 22‒36%); seven studies13,17,19-21,25,41 obtained a relatively higher survival (>37%), and six others14,18,26,32,35,37 reported significantly lower 5-year survival percentages (<21%).
Oral melanomas carry a worse prognosis than cutaneous melanomas due to their propensity for local invasion and distant metastasis. This is primarily attributed to delayed diagnosis, atypical clinical presentation, and continuous trauma related to their anatomical location, often resulting in ulcerated lesions as the disease progresses.44 Resection of primary oral melanoma lesions is the gold standard of treatment for these lesions. The main problem stems from the early metastatic nature of oral melanomas. Lymph node metastases are common, and neck dissection does not affect the development of future distant metastases or OS. Survival of patients with oral melanoma varies according to the tumor site and decreases when brain or liver metastases are present. The primary site of oral melanoma location, bone invasion, resection margins, depth of tumor invasion, and distant metastases are critical factors in predicting prognosis. They should be considered when selecting the most appropriate therapeutic option for treating oral mucosal melanoma.10 Other factors with a relevant influence on oral melanoma survival are the type of melanoma, with amelanotic melanomas having the worst prognosis probably due to diagnostic delay32 or the overexpression of certain genes such as the BAP1 gene that regulates cell differentiation, division, and death.34 The BAP1 gene encodes a tumor suppressor protein that plays a pivotal role in the pathogenesis of uveal melanoma. However, its relevance in oral melanomas remains poorly understood. In uveal melanoma, high BAP1 expression has been significantly associated with a favorable clinical prognosis. Conversely, in oral melanomas, BAP1 overexpression appears to be linked to an adverse prognosis and decreased OS. Evidence suggests that BAP1 inactivation in oral mucosal melanomas may play a role in tumor progression by promoting a more aggressive biological phenotype and reduced patient survival.45 Similarly, overexpression of heparanase, an enzyme released in the metabolism of tumor cells, also leads to poorer survival in oral melanomas, as they have a worse biological behavior.39 In oral mucosal melanomas, the transition from the radial growth phase to the invasive phase represents a critical step in tumor progression, as malignant cells migrate and infiltrate the underlying connective tissue. This process involves the synthesis and release of enzymes required for extracellular matrix degradation, such as heparanase.46
In the present study, melanomas located on the palate had a higher probability of 5-year survival, although statistical significance was not reached (P=0.41). Two39,40 of the three studies that considered tumor location coincided in indicating the palatal location, while the other one20 did not. This longer survival in tumors located in the palate could be conditioned by the achievement of negative resection margins at the time of surgery. This is much more difficult in other melanoma sites, such as the pharynx or paranasal sinus.20
Although women had a longer 5-year survival than men in this work, the results were also not statistically significant (P=0.13). Of the five studies that focused on the patient’s gender, four12,20,25,35 confirmed this longer survival in females, while for one,39 it was longer in males. The reason for the shorter survival of males with oral melanoma may lie in the higher incidence of this malignant tumor in males. The actual influence of gender on the biological behavior of oral mucosal melanomas remains to be elucidated.39 Women’s greater awareness of health problems may lead to an earlier diagnosis of oral melanoma and a longer survival time.25
In the present work, non-ulcerated melanomas increased the probability of 5-year survival by 3.78 times, with highly significant statistical differences (P<0.001). All studies12,33,34,40 that further examined this parameter corroborated this increased survival in non-ulcerated lesions. Ulceration is a clinical sign of long-term lesions found in the more advanced stages (III or IV) of the disease. These melanomas have a higher degree of invasion and deep infiltration that drastically decreases survival.40
In this study, patients with more pigmented melanomas were 2.47 times more likely to be alive at 5-year follow-up, with a highly significant statistical association (P<0.001). All studies13,33-35,39 that looked at the degree of pigmentation agreed that the more pigmented the melanoma, the higher the probability of 5-year survival. Due to their clinical presentation as red-like lesions, poorly pigmented melanomas (amelanotic melanomas) present delays in diagnosis. There is often diagnostic confusion with lesions of inflammatory-infectious origin. The diagnosis is made later, as they are metastatic lesions with a worse prognosis.13
Limitations
This study had some limitations. It was not possible to properly analyze certain parameters that significantly influence survival in oral melanomas, such as the evolution time of the tumor lesion or the presence of lymph nodes and distant metastases. Nor was it possible to evaluate the impact on survival of the different treatment alternatives of oral melanomas. Finally, the significant variability between studies may have conditioned the results, requiring a cautious interpretation.
Conclusion
In this meta-analysis, the OS of oral mucosal melanoma patients decreased from 58% at two years to 42% at three years and 29% at five years. Considering the prognostic factors, non-ulcerated oral melanomas with a high degree of pigmentation showed the highest 5-year survival. In contrast, oral tumor location and gender did not significantly affect oral melanoma survival.
Competing Interests
None.
Ethical Approval
Granada University Stomatology Department Scientific Committee (code EST/UGR/19-2024).
References
- McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol 2008; 44(11):1039-46. doi: 10.1016/j.oraloncology.2008.01.014 [Crossref] [ Google Scholar]
- Williams MD. Update from the 4th edition of the World Health Organization classification of head and neck tumours: mucosal melanomas. Head Neck Pathol 2017; 11(1):110-7. doi: 10.1007/s12105-017-0789-y [Crossref] [ Google Scholar]
- Berger DMS, Verver D, van der Noort V, Grünhagen DJ, Verhoef C, Al-Mamgani A. Therapeutic neck dissection in head and neck melanoma patients: comparing extent of surgery and clinical outcome in two cohorts. Eur J Surg Oncol 2021; 47(9):2454-9. doi: 10.1016/j.ejso.2021.04.007 [Crossref] [ Google Scholar]
- Lambertini M, Patrizi A, Fanti PA, Melotti B, Caliceti U, Magnoni C. Oral melanoma and other pigmentations: when to biopsy?. J Eur Acad Dermatol Venereol 2018; 32(2):209-14. doi: 10.1111/jdv.14574 [Crossref] [ Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses [Internet]. Ottawa, Canada: The Ottawa Hospital; 2000. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Wu AJ, Gomez J, Zhung JE, Chan K, Gomez DR, Wolden SL. Radiotherapy after surgical resection for head and neck mucosal melanoma. Am J Clin Oncol 2010; 33(3):281-5. doi: 10.1097/COC.0b013e3181a879f5 [Crossref] [ Google Scholar]
- Bakkal FK, Başman A, Kızıl Y, Ekinci Ö, Gümüşok M, Ekrem Zorlu M. Mucosal melanoma of the head and neck: recurrence characteristics and survival outcomes. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120(5):575-80. doi: 10.1016/j.oooo.2015.06.038 [Crossref] [ Google Scholar]
- Berthelsen A, Andersen AP, Jensen TS, Hansen HS. Melanomas of the mucosa in the oral cavity and the upper respiratory passages. Cancer 1984; 54(5):907-12. doi: 10.1002/1097-0142(19840901)54:5<907::aid-cncr2820540526>3.0.co;2-z [Crossref] [ Google Scholar]
- Breik O, Sim F, Wong T, Nastri A, Iseli TA, Wiesenfeld D. Survival outcomes of mucosal melanoma in the head and neck: case series and review of current treatment guidelines. J Oral Maxillofac Surg 2016; 74(9):1859-71. doi: 10.1016/j.joms.2016.03.008 [Crossref] [ Google Scholar]
- Chae YS, Lee JY, Lee JW, Park JY, Kim SM, Lee JH. Survival of oral mucosal melanoma according to treatment, tumour resection margin, and metastases. Br J Oral Maxillofac Surg 2020; 58(9):1097-102. doi: 10.1016/j.bjoms.2020.05.028 [Crossref] [ Google Scholar]
- Conley J, Pack GT. Melanoma of the mucous membranes of the head and neck. Arch Otolaryngol 1974; 99(5):315-9. doi: 10.1001/archotol.1974.00780030327001 [Crossref] [ Google Scholar]
- Francisco AL, Furlan MV, Peresi PM, Nishimoto IN, Lourenço SV, Pinto CA. Head and neck mucosal melanoma: clinicopathological analysis of 51 cases treated in a single cancer centre and review of the literature. Int J Oral Maxillofac Surg 2016; 45(2):135-40. doi: 10.1016/j.ijom.2015.08.987 [Crossref] [ Google Scholar]
- Guo W, Yin G, Liu H, Duan H, Huang Z, Chen X. Matched analysis of the prognosis of amelanotic and pigmented melanoma in head and neck. Acta Otolaryngol 2020; 140(9):785-8. doi: 10.1080/00016489.2020.1763456 [Crossref] [ Google Scholar]
- Jethanamest D, Vila PM, Sikora AG, Morris LG. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol 2011; 18(10):2748-56. doi: 10.1245/s10434-011-1685-4 [Crossref] [ Google Scholar]
- Lawaetz M, Birch-Johansen F, Friis S, Eriksen JG, Kiss K, Gade S. Primary mucosal melanoma of the head and neck in Denmark, 1982-2012: demographic and clinical aspects A retrospective DAHANCA study. Acta Oncol 2016; 55(8):1001-8. doi: 10.3109/0284186x.2016.1143117 [Crossref] [ Google Scholar]
- Lian B, Cui CL, Zhou L, Song X, Zhang XS, Wu D. The natural history and patterns of metastases from mucosal melanoma: an analysis of 706 prospectively-followed patients. Ann Oncol 2017; 28(4):868-73. doi: 10.1093/annonc/mdw694 [Crossref] [ Google Scholar]
- Meleti M, Leemans CR, de Bree R, Vescovi P, Sesenna E, van der Waal I. Head and neck mucosal melanoma: experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck 2008; 30(12):1543-51. doi: 10.1002/hed.20901 [Crossref] [ Google Scholar]
- Moore ES, Martin H. Melanoma of the upper respiratory tract and oral cavity. Cancer 1955; 8(6):1167-76. doi: 10.1002/1097-0142(1955)8:6<1167::aidcncr2820080613>3.0.co;2-i [Crossref] [ Google Scholar]
- Moya-Plana A, Aupérin A, Obongo R, Baglin A, Ferrand FR, Baujat B. Oncologic outcomes, prognostic factor analysis and therapeutic algorithm evaluation of head and neck mucosal melanomas in France. Eur J Cancer 2019; 123:1-10. doi: 10.1016/j.ejca.2019.09.007 [Crossref] [ Google Scholar]
- Naganawa K, Koto M, Takagi R, Hasegawa A, Ikawa H, Shimozato K. Long-term outcomes after carbon-ion radiotherapy for oral mucosal malignant melanoma. J Radiat Res 2017; 58(4):517-22. doi: 10.1093/jrr/rrw117 [Crossref] [ Google Scholar]
- Owens JM, Roberts DB, Myers JN. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch Otolaryngol Head Neck Surg 2003; 129(8):864-8. doi: 10.1001/archotol.129.8.864 [Crossref] [ Google Scholar]
- Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002; 24(3):247-57. doi: 10.1002/hed.10019 [Crossref] [ Google Scholar]
- Perri F, Pisconti S, Favia M, Della Vittoria Scarpati G, Conson M, Giuliano M. Optimal multidisciplinary treatment of oral cavity mucosal melanoma: outcome analysis in a case series. Anticancer Drugs 2017; 28(3):327-34. doi: 10.1097/cad.0000000000000454 [Crossref] [ Google Scholar]
- Prasad ML, Patel S, Hoshaw-Woodard S, Escrig M, Shah JP, Huvos AG. Prognostic factors for malignant melanoma of the squamous mucosa of the head and neck. Am J Surg Pathol 2002; 26(7):883-92. doi: 10.1097/00000478-200207000-00006 [Crossref] [ Google Scholar]
- Prinzen T, Klein M, Hallermann C, Wermker K. Primary head and neck mucosal melanoma: Predictors of survival and a case series on sentinel node biopsy. J Craniomaxillofac Surg 2019; 47(9):1370-7. doi: 10.1016/j.jcms.2019.06.012 [Crossref] [ Google Scholar]
- Rapini RP, Golitz LE, Greer RO Jr, Krekorian EA, Poulson T. Primary malignant melanoma of the oral cavity A review of 177 cases. Cancer 1985; 55(7):1543-51. doi: 10.1002/1097-0142(19850401)55:7<1543::aidcncr2820550722>3.0.co;2-f [Crossref] [ Google Scholar]
- Sahovaler A, Ziai H, Cardemil F, Huang SH, Su J, Goldstein DP. Importance of margins, radiotherapy, and systemic therapy in mucosal melanoma of the head and neck. Laryngoscope 2021; 131(10):2269-76. doi: 10.1002/lary.29555 [Crossref] [ Google Scholar]
- Schaefer T, Satzger I, Gutzmer R. Clinics, prognosis and new therapeutic options in patients with mucosal melanoma: a retrospective analysis of 75 patients. Medicine (Baltimore) 2017; 96(1):e5753. doi: 10.1097/md.0000000000005753 [Crossref] [ Google Scholar]
- Schmidt MQ, David J, Yoshida EJ, Scher K, Mita A, Shiao SL. Predictors of survival in head and neck mucosal melanoma. Oral Oncol 2017; 73:36-42. doi: 10.1016/j.oraloncology.2017.08.002 [Crossref] [ Google Scholar]
- Shah JP, Huvos AG, Strong EW. Mucosal melanomas of the head and neck. Am J Surg 1977; 134(4):531-5. doi: 10.1016/0002-9610(77)90393-2 [Crossref] [ Google Scholar]
- Shuman AG, Light E, Olsen SH, Pynnonen MA, Taylor JM, Johnson TM. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg 2011; 137(4):331-7. doi: 10.1001/archoto.2011.46 [Crossref] [ Google Scholar]
- Soares CD, Carlos R, de Andrade BA, Cunha JL, Agostini M, Romañach MJ. Oral amelanotic melanomas: clinicopathologic features of 8 cases and review of the literature. Int J Surg Pathol 2021; 29(3):263-72. doi: 10.1177/1066896920946435 [Crossref] [ Google Scholar]
- Song H, Jing G, Wang L, Guo W, Ren G. Periodic acid-Schiff-positive loops and networks as a prognostic factor in oral mucosal melanoma. Melanoma Res 2016; 26(2):145-52. doi: 10.1097/cmr.0000000000000220 [Crossref] [ Google Scholar]
- Song H, Wang L, Lyu J, Wu Y, Guo W, Ren G. Loss of nuclear BAP1 expression is associated with poor prognosis in oral mucosal melanoma. Oncotarget 2017; 8(17):29080-90. doi: 10.18632/oncotarget.16175 [Crossref] [ Google Scholar]
- Sun CZ, Chen YF, Jiang YE, Hu ZD, Yang AK, Song M. Treatment and prognosis of oral mucosal melanoma. Oral Oncol 2012; 48(7):647-52. doi: 10.1016/j.oraloncology.2012.01.019 [Crossref] [ Google Scholar]
- Tanaka N, Mimura M, Ogi K, Amagasa T. Primary malignant melanoma of the oral cavity: assessment of outcome from the clinical records of 35 patients. Int J Oral Maxillofac Surg 2004; 33(8):761-5. doi: 10.1016/j.ijom.2004.01.008 [Crossref] [ Google Scholar]
- Temam S, Mamelle G, Marandas P, Wibault P, Avril MF, Janot F. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005; 103(2):313-9. doi: 10.1002/cncr.20775 [Crossref] [ Google Scholar]
- Trodahl JN, Sprague WG. Benign and malignant melanocytic lesions of the oral mucosa An analysis of 135 cases. Cancer 1970; 25(4):812-23. doi: 10.1002/1097-0142(197004)25:4<812::aidcncr2820250410>3.0.co;2-g [Crossref] [ Google Scholar]
- Wang X, Wen W, Wu H, Chen Y, Ren G, Guo W. Heparanase expression correlates with poor survival in oral mucosal melanoma. Med Oncol 2013; 30(3):633. doi: 10.1007/s12032-013-0633-5 [Crossref] [ Google Scholar]
- Wu Y, Wang L, Ma X, Guo W, Ren G. The existence of early stage oral mucosal melanoma: a 10-year retrospective analysis of 170 patients in a single institute. Oral Oncol 2018; 87:70-6. doi: 10.1016/j.oraloncology.2018.10.022 [Crossref] [ Google Scholar]
- Yamada SI, Kurita H, Kamata T, Kirita T, Ueda M, Yamashita T. Clinical investigation of 38 cases of oral mucosal melanoma: a multicentre retrospective analysis in Japan. Australas J Dermatol 2017; 58(4):e223-7. doi: 10.1111/ajd.12603 [Crossref] [ Google Scholar]
- Yang X, Ren GX, Zhang CP, Zhou GY, Hu YJ, Yang WJ. Neck dissection and post-operative chemotherapy with dimethyl triazeno imidazole carboxamide and cisplatin protocol are useful for oral mucosal melanoma. BMC Cancer 2010; 10:623. doi: 10.1186/1471-2407-10-623 [Crossref] [ Google Scholar]
- Yii NW, Eisen T, Nicolson M, A’Hern R, Rhys-Evans P, Archer D. Mucosal malignant melanoma of the head and neck: the Marsden experience over half a century. Clin Oncol (R Coll Radiol) 2003; 15(4):199-204. doi: 10.1016/s0936-6555(03)00068-2 [Crossref] [ Google Scholar]
- Viscardi JA, Scampa M, Walz SN, Martineau J, di Summa PG, Giordano S. Cutaneous melanoma of the lip: a SEER analysis of epidemiology and survival outcomes with focus on surgery and other treatment options. In Vivo 2023; 37(3):1164-72. doi: 10.21873/invivo.13191 [Crossref] [ Google Scholar]
- Wang T, Zhu T, Zhang Y, Bai J, Xue Y, Xu G. Pan-cancer analysis of the prognostic and immunological role of BRCA1-associated protein 1 gene (BAP1): friend or foe?. Gene 2022; 840:146765. doi: 10.1016/j.gene.2022.146765 [Crossref] [ Google Scholar]
- Rivera RS, Nagatsuka H, Siar CH, Gunduz M, Tsujigiwa H, Han PP. Heparanase and vascular endothelial growth factor expression in the progression of oral mucosal melanoma. Oncol Rep 2008; 19(3):657-61. [ Google Scholar]