J Dent Res Dent Clin Dent Prospects. 19(1):40-45.
doi: 10.34172/joddd.025.41648
Original Article
Correlation between vitamin D3 serum levels and jaw bone density of candidates for dental implant treatment using CBCT
Ali Mesgarzadeh Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing, 1 
Amir Zandesh Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, 2, * 
Hooman Sojoudi Data curation, Investigation, Writing – original draft, Writing – review & editing, 3 
Ali Hossein Mesgarzadeh Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing, 3 
Farzad Esmaeili Formal analysis, Methodology, Supervision, Validation, Writing – review & editing, 4 
Emran Hajmohammadi Methodology, Project administration, Supervision, Validation, Writing – review & editing, 5 
Author information:
1Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
2Student of Dentistry, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Oral and Maxillofacial Radiology, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ardabil University of Medical Sciences, Ardabil, Iran
Abstract
Background.
Little information is available about the effect of vitamin D on jaw bone density, and human studies about this entity are scarce. Vitamin D deficiency weakens bone regeneration and is responsible for many systemic diseases, such as chronic kidney disease, diabetes mellitus, etc.
Methods.
Fifty candidates for dental implant treatment aged 20‒60 were randomly chosen at the Implantology Department of the Faculty of Dentistry, Tabriz University of Medical Sciences. Fifteen patients were male, and 35 were female, and they were examined for their vitamin D3 serum levels. All the patients had cone-beam computed tomography (CBCT) radiographic images. According to their vitamin D3 levels, they were split into three groups: deficient, insufficient, and sufficient, and the density of bone was evaluated using the mean calculated Hounsfield unit from the Planmeca Romexis software.
Results.
In the vitamin D3-deficient group, the mandibular bone of both males and females demonstrated lower bone densities; however, there was no significant correlation between bone density and vitamin D3 serum levels in either the maxilla or the mandible.
Conclusion.
This study could not find a correlation between the serum levels of vitamin D3 and the bone density of the jaws. Further studies are necessary in this respect.
Keywords: Bone density, CBCT, Dental implants, Vitamin D3
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Introduction
Vitamin D has a distinctive role in human health, survival, and fertility. Several studies have emphasized its role in preventing various pathologic conditions, including cardiovascular diseases, malignant conditions, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, type 1 diabetes, immune system disorders, and infectious processes. Additionally, calcium and phosphorus absorption is improved by vitamin D, the two most essential ions of bone, from the intestines, reducing their excretion from the kidneys and strengthening bone formation. Vitamin D deficiency is considered one of the critical factors in bone metabolism disorders.1,2
Measuring bone density can serve as a predictive sign for fractures resulting from osteoporosis. However, vitamin D significantly impacts the metabolism of calcium and phosphorus. The amount of 25-hydroxyvitamin D in the blood is well recognized as an indicator of a person’s vitamin D status.3,4
Wical and Brussee reported that patients taking vitamin D supplements experienced significantly less bone loss after getting immediate dentures compared to those who did not take supplements.5
The development of dental implants to achieve osseointegration has become a widely used method for restoring oral function. Knowing the Hounsfield number as a method of bone density measurements for implant placement can help surgeons adopt appropriate treatment plans for patients with poor bone density.6,7
Bazal-Bonelli et al,8 in their systematic review of the relationship between serum vitamin D levels and dental implants in terms of marginal bone loss, survival rates, and associated complications, found that cases with lower serum vitamin D levels were associated with slightly worse outcomes in terms of marginal bone loss.
Recent research on the alveolar ridge and healing of freshly extracted tooth areas indicates that calcium and cholecalciferol (vitamin D3) supplements have systemic effects that accelerate bone regeneration. The quantity and quality of bone available at the implant placement site strongly affects the success rate of dental implant treatment. Studies show that in cases where bone quantity and quality are inadequate, the failure rate of implant treatment rises.9,10 Due to the rising demand for dental implants, their dependence on healthy bone structure, and the impact of vitamin D3 deficiency on bone, this study aims to measure serum levels of vitamin D and bone density in referring patients to the Dental Implant Department of Tabriz Faculty of Dentistry.
Methods
This cross-sectional study included 50 patients visiting the Dental Implant Department of the Tabriz Faculty of Dentistry.
According to different sources,11 vitamin D deficiency was defined as having a vitamin D serum level < 20 ng/mL, while serum levels < 12 ng/mL are classified as severe deficiency.
Inclusion criteria
All patients who were candidates for implant treatment in the posterior alveolar region, required cone-beam computed tomography (CBCT) imaging, and were willing to take part completed a written informed consent form at the start of the study.
Exclusion criteria
Patients who did not want a serum level of vitamin D3 test, patients suffering from a systemic disease related to bone metabolism, patients taking vitamin D3 supplements, and post-menopause women were excluded.
Patients who were candidates for implantation in the posterior alveolar areas and had CBCT images were selected randomly. They were ordered a free serum vitamin D3 test. The test was conducted in a private facility using a standard kit and measured by one person.
A trained radiologist implemented the Hounsfield number to quantify the bone density in the posterior edentulous zones extending from the first premolar to the wisdom teeth. All the patients were examined using a CBCT machine (Newtom VGi) at Tabriz Faculty of Dentistry.
Bone density was measured about one millimeter away from the inferior alveolar canal and the maxillary sinuses to avoid these structures. Only the trabecular bone was included in the measurements, and the cortical bone was excluded to ensure consistent results.
A specific measurement protocol was employed in the Romexis Planmeca software. The defined measurement area was a uniform mass of 3 × 3 mm. Within this area, the software automatically calculated the average Hounsfield numbers (as a measure of radiodensity) and presented them as an average value (Figures 1 and 2).
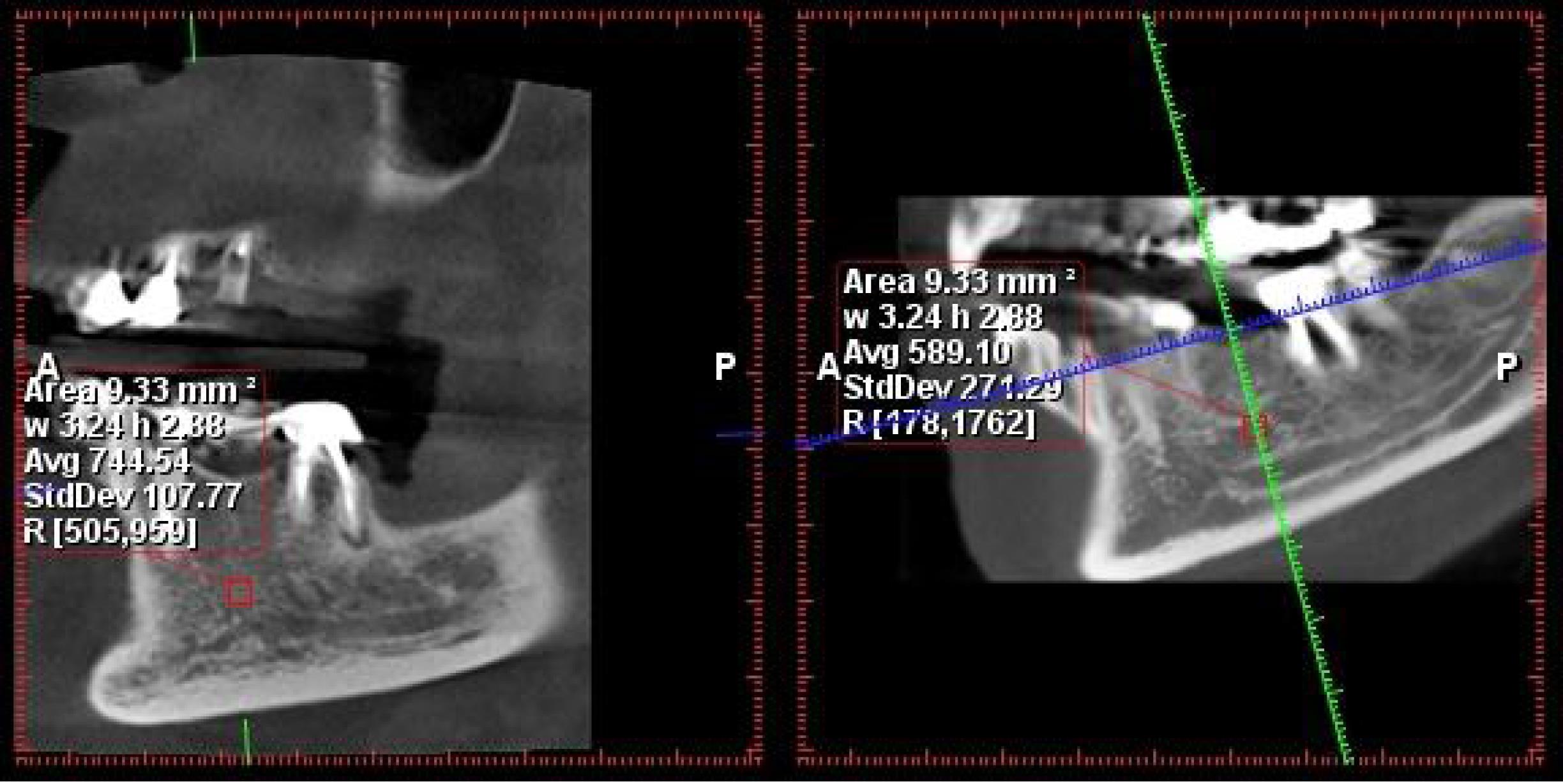
Figure 1.
The measurement of bone density in the posterior mandibular region
.
The measurement of bone density in the posterior mandibular region
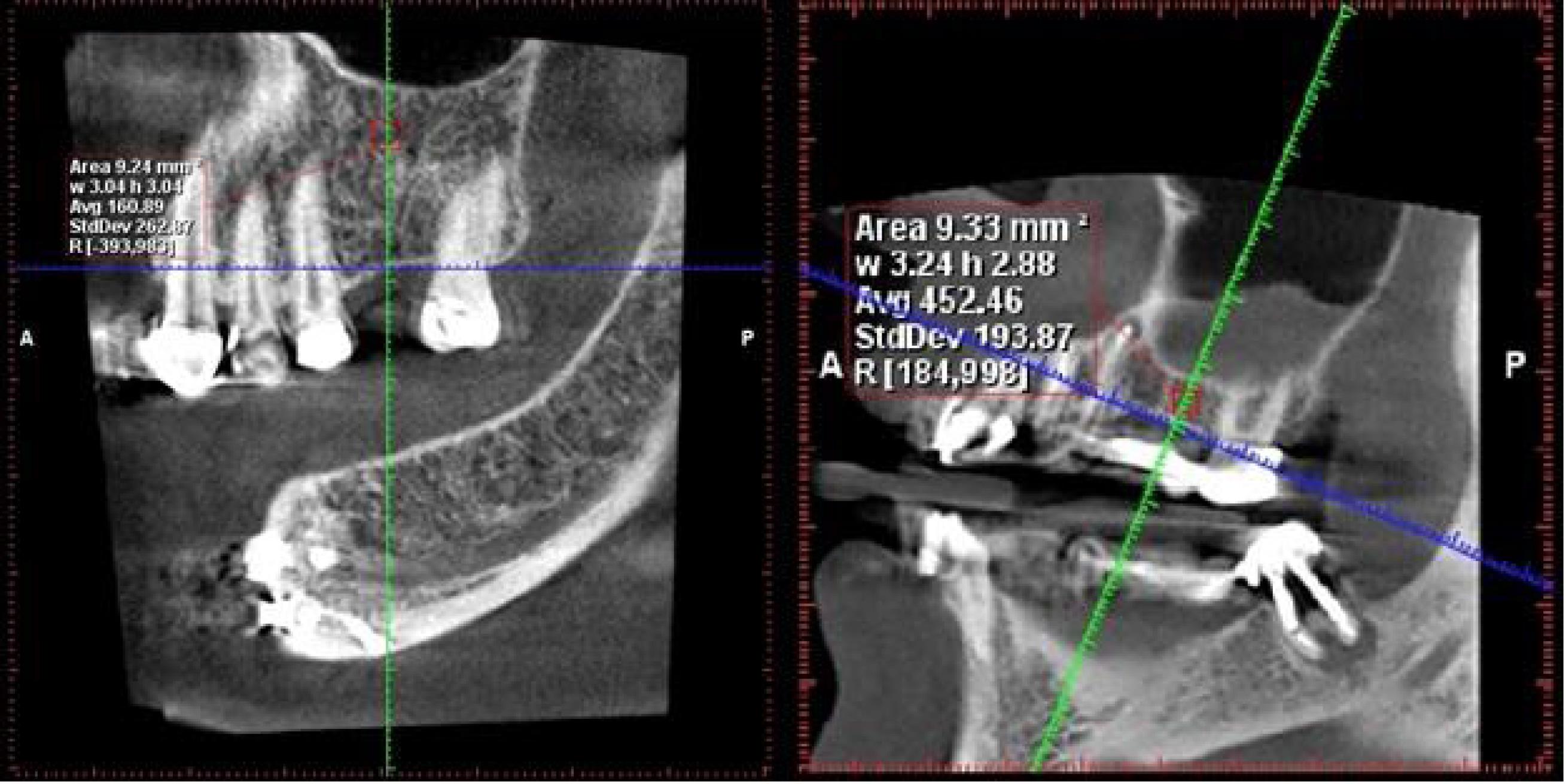
Figure 2.
The measurement of bone density in the posterior maxilla region
.
The measurement of bone density in the posterior maxilla region
This average value was typically obtained from the edentulous areas corresponding to tooth numbers #6 and #5. If those areas were not available, measurements were taken from other posterior regions. Notably, the measurements were made at a level with a 1-mm distance from both the inferior alveolar canal and the maxillary sinus floor.
The findings were presented as means ± standard deviations (SD) and percentages. The Kruskal-Wallis test was used for comparison, and the Kolmogorov-Smirnov test was used to determine whether the data was normal. SPSS 24 was used for statistical analyses.
A result was considered statistically significant if the P value was < 0.05.
Results
This study investigated the effect of vitamin D serum level on the density of jaw bones in 50 patients.
In this study, 30% of patients were male and 70% were female (Table 1). The findings from Table 2 indicate that the mean vitamin D3 serum level in the studied patients was 30.12 ± 11.95 ng/mL, with a range of 4‒65.2 ng/mL in serum. Additionally, Table 3 shows that 22% of the patients had vitamin D3 deficiency, 18% had vitamin D3 insufficiency, and 60% had normal vitamin D3 levels. Bone density analysis showed an average value of 372.37 ± 143.88 in the maxilla and 436.21 ± 184.34 in the mandible (Table 4).
Table 1.
The frequencies of examined patients by gender
|
|
Number
|
Percent
|
| Men |
15 |
30.0 % |
| Women |
35 |
70.0 % |
| Total |
50 |
100.0 % |
Table 2.
Means and standard deviations and minimum and maximum amounts of vitamin D3 in the examined patients
|
|
Number
|
Minimum
|
Maximum
|
Mean
|
SD
|
| Vitamin D3 |
50 |
4.00 |
65.20 |
30.12 |
11.95 |
Table 3.
A comparison of patient frequencies according to body levels of vitamin D3
|
|
Number
|
Percent
|
| Deficiency |
11 |
22.0 |
| Insufficient |
9 |
18.0 |
| Normal |
30 |
60.0 |
| Total |
50 |
100.0 |
Deficiency: < 20 ng/mL vitamin D3 serum level.
Insufficient: 20‒30 ng/mL vitamin D3 serum level.
Normal: > 30 ng/mL vitamin D3 serum level.
Table 4.
Means and standard deviations and minimum and maximum values of bone density in the maxilla and mandible in the examined patients
|
|
Number
|
Minimum
|
Maximum
|
Mean
|
SD
|
| Maxilla |
39 |
144.00 |
733.50 |
372.37 |
143.88 |
| Mandible |
38 |
115.50 |
800.50 |
436.21 |
184.34 |
This study revealed no significant difference in bone density between the maxilla and mandible in male patients across various serum levels of vitamin D3. There was a slight increase in bone density in the mandible with higher vitamin D3 levels, but it was not statistically significant. Similarly, in female patients, across different vitamin D3 levels, there was no significant difference in the maxilla and mandible’s bone density. However, women with vitamin D3 deficiency had the lowest bone density in both the maxilla and mandible, though this finding was not statistically significant (Tables 5 and 6).
Table 5.
Comparison of bone density based on vitamin D3 group in both jaws and in men and women (P value: Kruskal-Wallis)
|
|
Vitamin D3
|
Men
|
Women
|
|
Number
|
Mean
|
SD
|
Number
|
Mean
|
SD
|
| Maxilla |
Deficiency |
4 |
470.50 |
248.71 |
4 |
270.13 |
142.38 |
| Insufficient |
3 |
399.67 |
167.69 |
4 |
462.13 |
96.97 |
| Normal |
3 |
446.17 |
27.59 |
20 |
351.50 |
117.49 |
| Total |
10 |
441.95 |
167.34 |
28 |
355.68 |
125.65 |
|
P value |
0.885 |
0.089 |
| Mandible |
Deficiency |
5 |
464.70 |
87.96 |
5 |
216.60 |
108.11 |
| Insufficient |
4 |
576.00 |
168.68 |
2 |
443.25 |
22.27 |
| Normal |
3 |
630.50 |
119.62 |
19 |
425.66 |
186.53 |
| Total |
12 |
543.25 |
135.81 |
26 |
386.81 |
184.76 |
|
P value |
0.220 |
0.066 |
SD: standard deviation.
Deficiency: < 20 ng/mL vitamin D3 serum level.
Insufficient: 30-20 ng/mL vitamin D3 serum level.
Normal: > 30 ng/mL vitamin D3 serum level.
Table 6.
Comparison of bone density based on vitamin D3 group in two jaws (P value: Kruskal-Wallis)
|
Vitamin D3
|
Maxilla
|
Mandible
|
|
Number
|
Mean
|
SD
|
Number
|
Mean
|
SD
|
| Deficiency |
8 |
370.31 |
216.03 |
10 |
340.65 |
160.41 |
| Insufficient |
7 |
435.36 |
123.25 |
6 |
531.75 |
147.88 |
| Normal |
23 |
363.85 |
114.26 |
22 |
453.59 |
190.69 |
| Total |
38 |
378.38 |
140.76 |
38 |
436.21 |
184.34 |
|
P value |
0.505 |
0.104 |
SD: standard deviation.
Deficiency: < 20 ng/mL vitamin D3 serum level.
Insufficient: 30-20 ng/mL vitamin D3 serum level.
Normal: > 30 ng/mL vitamin D3 serum level.
Discussion
The quality and quantity of bone influence dental implant treatment success in the implant placement area. Previous studies have shown that implant failure rates increase in bones with poor quantity and quality.7
In the present study, 50 patients underwent implant surgery; 30% of patients were male, and 70% were female. 22% of patients had vitamin D3 deficiency, 18% were in the insufficient range, and 60% were in the normal range. Bone density in both the maxilla and mandible showed no significant relationship with serum vitamin D3 levels. Also, there was no significant difference in the average bone density in different vitamin D3 groups.
Werny et al, in a systematic review of animal and human studies, indicated that lower vitamin D serum levels negatively affect implant osseointegration in animals. In animals with systemic disorders such as osteoporosis, diabetes mellitus, chronic renal disease, and vitamin D deficiency, vitamin D supplements improve osseointegration. There is some evidence to support the theory that vitamin D improves osseointegration in humans in a similar way. The results of this study contradict the present study.12
Acipinar et al,13 in a study of 90 dental implant sites, evaluated the peri-implant sulcus fluid 25-hydroxy-vitamin D3 (25(OH)D3) levels in peri-implant healthy tissues and peri-implant diseases and indicated that the 25(OH)D3 concentration was significantly lower in the peri-implantitis group. Similarly, Singh et al14 found a positive correlation between vitamin D serum levels and crestal bone loss on CBCT in their investigation of the relationship between serum vitamin D and crestal bone level in dental implant patients using CBCT.
In a retrospective study in 2016, Mangano et al15 investigated the association of vitamin D serum level with early implant failure in 1625 implants. Although that study reported an increasing trend in the incidence of primary implant failure with vitamin D deficiency, there was no significant difference in the frequency of primary implant failure across the three vitamin D groups studied, consistent with the present investigation.
Examining the serum level of vitamin D3 in patients depends on factors such as age, gender, body mass index, exposure to sunlight, skin pigmentation severity, and type of nutrition.16-18 These factors can act as confounding variables in the current study and similar studies. However, in the present study, some controllable factors, such as the patients consuming vitamin D3 supplements and women after menopause, were excluded.
Research has shown that vitamin D stimulates the activity of bone cells, and in patients who have received enough vitamin D, bone density is higher, and bone fractures are fewer.19 Conversely, vitamin D is essential in regulating the dynamic state of bone and controlling the activity of bone cells like osteoblasts and osteoclasts. Researchers have shown that vitamin D has an anabolic effect on osteoblasts.20 Consequently, a recent trend in the field of dental implant success is to promote immune system regulation to achieve quicker and more effective osseointegration.21,22 Therefore, vitamin D stimulates osteoblasts to synthesize several factors that promote osseointegration and bone formation.23
Schulze-Späte et al24 showed in a 2016 clinical trial that vitamin D3 plus calcium raises serum vitamin D levels and affects bone regeneration to improve maxillary sinus augmentation. Nevertheless, there were no statistically significant differences in graft resorption or bone growth between the supplement therapy and control groups.
In a 2015 study on the regenerative response of alveolar bone to topical calcitriol application in vitamin D-deficient rats, Fügl et al25 demonstrated that topical calcitriol application does not promote bone healing and that vitamin D deficiency does not always have a negative effect on bone regeneration in the rat jaw.
In a review of animals, Javed et al26 showed five studies that indicated the significant effect of vitamin D3 supplementation on increasing new bone formation or bone-implant contact around the implants.
In the present study, bone density was evaluated using CBCT images. CBCT images use a reduced radiation dose and are one way to evaluate bone quality before placing an implant.
Shapurian et al6 showed that Hounsfield values, as a quantitative indicator of bone density, could be a useful diagnostic tool, provide an objective assessment of bone density for the implant surgeon, and lead to the modification of surgical procedures, especially in suspected conditions of low bone quality.
In the 2014 study by Razi et al,27 the relationship between grayscale in CBCT and HU in CT was examined. It showed that the grayscale in CBCT is a criterion for assessing bone density before implant treatment, and due to low radiation and the low cost of CBCT, it is the method of choice compared to CT scan.
According to the current study, clinical studies with higher sample sizes are recommended to reach a definite conclusion about the relationship between the serum levels of vitamin D and bone density.
Conclusion
This study found no significant relationship between serum vitamin D3 levels and bone density in the maxillary and mandibular regions. The bone density of men and women in the various vitamin D3 groups did not differ significantly. However, in the mandible, the lowest bone density was observed in patients with vitamin D3 deficiency.
Competing Interests
The authors deny any conflict of interests related to this study.
Ethical Approval
The present study was approved by the Ethics Committee of Tabriz University of Medical Sciences under the code IR.TBZMED.REC.1398.1267.
References
- Urena-Torres P, Souberbielle JC. Pharmacologic role of vitamin D natural products. Curr Vasc Pharmacol 2014; 12(2):278-85. doi: 10.2174/15701611113119990020 [Crossref] [ Google Scholar]
- Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther 2010; 8(12):1359-69. doi: 10.1586/eri.10.102 [Crossref] [ Google Scholar]
- Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi: 10.1136/bmj.h4580 [Crossref] [ Google Scholar]
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014; 383(9912):146-55. doi: 10.1016/s0140-6736(13)61647-5 [Crossref] [ Google Scholar]
- Wical KE, Brussee P. Effects of a calcium and vitamin D supplement on alveolar ridge resorption in immediate denture patients. J Prosthet Dent 1979; 41(1):4-11. doi: 10.1016/0022-3913(79)90347-0 [Crossref] [ Google Scholar]
- Shapurian T, Damoulis PD, Reiser GM, Griffin TJ, Rand WM. Quantitative evaluation of bone density using the Hounsfield index. Int J Oral Maxillofac Implants 2006; 21(2):290-7. [ Google Scholar]
- Turkyilmaz I, Tumer C, Ozbek EN, Tözüm TF. Relations between the bone density values from computerized tomography, and implant stability parameters: a clinical study of 230 regular platform implants. J Clin Periodontol 2007; 34(8):716-22. doi: 10.1111/j.1600-051X.2007.01112.x [Crossref] [ Google Scholar]
- Bazal-Bonelli S, Sánchez-Labrador L, Cortés-Bretón Brinkmann J, Cobo-Vázquez C, Martínez-Rodríguez N, Beca-Campoy T. Influence of serum vitamin D levels on survival rate and marginal bone loss in dental implants: a systematic review. Int J Environ Res Public Health 2022; 19(16):10120. doi: 10.3390/ijerph191610120 [Crossref] [ Google Scholar]
- de Elío Oliveros J, Del Canto Díaz A, Del Canto Díaz M, Orea CJ, Del Canto Pingarrón M, Calvo JS. Alveolar bone density and width affect primary implant stability. J Oral Implantol 2020; 46(4):389-95. doi: 10.1563/aaid-joi-D-19-00028 [Crossref] [ Google Scholar]
- Hong HH, Chou TA, Yang JC, Chang CJ. The potential effects of cholecalciferol on bone regeneration in dogs. Clin Oral Implants Res 2012; 23(10):1187-92. doi: 10.1111/j.1600-0501.2011.02284.x [Crossref] [ Google Scholar]
- Jeong SH, Kim JS, Kim HJ, Choi JY, Koo JW, Choi KD. Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: a randomized trial. Neurology 2020; 95(9):e1117-25. doi: 10.1212/wnl.0000000000010343 [Crossref] [ Google Scholar]
- Werny JG, Sagheb K, Diaz L, Kämmerer PW, Al-Nawas B, Schiegnitz E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int J Implant Dent 2022; 8(1):16. doi: 10.1186/s40729-022-00414-6 [Crossref] [ Google Scholar]
- Acipinar S, Karsiyaka Hendek M, Olgun E, Kisa U. Evaluation of FGF-23 and 25(OH)D3 levels in peri-implant sulcus fluid in peri-implant health and diseases. Clin Implant Dent Relat Res 2019; 21(5):1106-12. doi: 10.1111/cid.12832 [Crossref] [ Google Scholar]
- Singh S, Dhawan P, Kaur H. Correlation of serum vitamin D with crestal bone level in dental implant patients using CBCT: a clinical retrospective study. J Contemp Dent Pract 2023; 24(7):415-8. doi: 10.5005/jp-journals-10024-3537 [Crossref] [ Google Scholar]
- Mangano F, Mortellaro C, Mangano N, Mangano C. Is low serum vitamin D associated with early dental implant failure? A retrospective evaluation on 1625 implants placed in 822 patients. Mediators Inflamm 2016; 2016:5319718. doi: 10.1155/2016/5319718 [Crossref] [ Google Scholar]
- Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc 2005; 105(6):971-4. doi: 10.1016/j.jada.2005.03.002 [Crossref] [ Google Scholar]
- Talaei A, Yadegari N, Rafee M, Rezvanfar MR, Moini A. Prevalence and cut-off point of vitamin D deficiency among secondary students of Arak, Iran in 2010. Indian J Endocrinol Metab 2012; 16(5):786-90. doi: 10.4103/2230-8210.100676 [Crossref] [ Google Scholar]
- Nackaerts O, Maes F, Yan H, Couto Souza P, Pauwels R, Jacobs R. Analysis of intensity variability in multislice and cone beam computed tomography. Clin Oral Implants Res 2011; 22(8):873-9. doi: 10.1111/j.1600-0501.2010.02076.x [Crossref] [ Google Scholar]
- Anderson PH, Sawyer RK, May BK, O’Loughlin PD, Morris HA. 25-Hydroxyvitamin D requirement for maintaining skeletal health utilising a Sprague-Dawley rat model. J Steroid Biochem Mol Biol 2007; 103(3-5):592-5. doi: 10.1016/j.jsbmb.2006.12.086 [Crossref] [ Google Scholar]
- Chang PL, Prince CW. 1 alpha,25-Dihydroxyvitamin D3 enhances 12-O-tetradecanoylphorbol-13-acetate- induced tumorigenic transformation and osteopontin expression in mouse JB6 epidermal cells. Cancer Res 1993; 53(10 Suppl):2217-20. [ Google Scholar]
- Jafarpour Mahalleh A, Mesgarzadeh AH, Jarolmasjed S, Soltani Somee A, Khordadmehr M, Rezaei Y. Extracorporeal shock wave therapy as a helpful method for rapid osseointegration of dental implants: animal study. Biomimetics (Basel) 2023; 8(2):137. doi: 10.3390/biomimetics8020137 [Crossref] [ Google Scholar]
- Albrektsson T, Sennerby L. Direct bone anchorage of oral implants: clinical and experimental considerations of the concept of osseointegration. Int J Prosthodont 1990; 3(1):30-41. [ Google Scholar]
- Buzatu BLR, Buzatu R, Luca MM. Impact of vitamin D on osseointegration in dental implants: a systematic review of human studies. Nutrients 2024; 16(2):209. doi: 10.3390/nu16020209 [Crossref] [ Google Scholar]
- Schulze-Späte U, Dietrich T, Wu C, Wang K, Hasturk H, Dibart S. Systemic vitamin D supplementation and local bone formation after maxillary sinus augmentation - a randomized, double-blind, placebo-controlled clinical investigation. Clin Oral Implants Res 2016; 27(6):701-6. doi: 10.1111/clr.12641 [Crossref] [ Google Scholar]
- Fügl A, Gruber R, Agis H, Lzicar H, Keibl C, Schwarze UY. Alveolar bone regeneration in response to local application of calcitriol in vitamin D deficient rats. J Clin Periodontol 2015; 42(1):96-103. doi: 10.1111/jcpe.12342 [Crossref] [ Google Scholar]
- Javed F, Malmstrom H, Kellesarian SV, Al-Kheraif AA, Vohra F, Romanos GE. Efficacy of vitamin D3 supplementation on osseointegration of implants. Implant Dent 2016; 25(2):281-7. doi: 10.1097/id.0000000000000390 [Crossref] [ Google Scholar]
- Razi T, Niknami M, Alavi Ghazani F. Relationship between Hounsfield unit in CT scan and gray scale in CBCT. J Dent Res Dent Clin Dent Prospects 2014; 8(2):107-10. doi: 10.5681/joddd.2014.019 [Crossref] [ Google Scholar]