J Dent Res Dent Clin Dent Prospects. 17(4):227-234.
doi: 10.34172/joddd.2023.40536
Original Article
Effect of air abrasive polishing on nickel release, stainless steel corrosion, and nickel-titanium archwires
Mohanad Ali Mohammed Data curation, Investigation, Methodology, Project administration, Visualization, Writing – original draft, * 
Alan Issa Saleem Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing,
Author information:
Department of Orthodontics, College of Dentistry, University of Baghdad, Bab Al-Muadham Campus, Baghdad, Iraq
Abstract
Background.
Orthodontic treatment is becoming more and more popular. However, using fixed orthodontic devices for treatment affects oral hygiene and raises the risk of corrosion, plaque-related illnesses, and dental discoloration-related issues. Air abrasive polishing has a superior effect over the conventional method in removing dental deposits. Using fixed orthodontic appliances affects oral hygiene and raises the risk of diseases caused by plaque, tooth discoloration, and corrosion, as well as corrosion by ions. This study evaluated the impact of air polishing on nickel ion release and corrosion from stainless steel, nickel-titanium, coated stainless steel, and coated nickel-titanium.
Methods.
A total of 288 (stainless steel, coated stainless-steel, nickel-titanium, and coated nickel-titanium rectangular archwires) of one brand were subjected to varying air abrasion polishing times (5, 10, and 20 seconds). Then, they were submerged in artificial saliva with a pH of 6.75 and incubated for 28 days at 37 °C. The release of nickel ions (Ni2+) was measured using an atomic absorption spectrophotometer at 7, 14, and 28 days to estimate the cumulative effect. The corrosion of the test-selected samples and surface alterations was evaluated using scanning electron microscopy (SEM).
Results.
Prolonged polishing significantly increased Ni2+ release and corrosion. Archwires made of coated stainless steel exhibited the least amount of Ni2+ release.
Conclusion.
The air polishing process increased the Ni2+ release at a subtoxic level and could be used on adult patients with long gaps between visits with a polishing period of 5 seconds.
Keywords: Abrasive polishing, Archwires, Nickle, Stainless steel, Titanium
Copyright and License Information
©2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Introduction
The need for fixed orthodontic appliances has increased. However, there might be several drawbacks during orthodontic treatment, like plaque-related allergies and conditions. The unfavorable oral environment, characterized by the presence of bacteria and other microorganisms, can act as a favorable medium for the electrochemical corrosion of embedded metallic objects such as braces, wires, and accessories because the microorganisms present in the oral cavity can produce electrolytes and organic acids that can corrode the metal. In addition, the high pH levels in the oral environment can increase the corrosion rate.1 Furthermore, metal orthodontic components may experience increased metallic corrosion when exposed to harmful physical and chemical contaminants.2 Investigations into the potential mutagenic, allergenic, and carcinogenic effects of the released ions from the corrosion process of the metallic alloys used to create orthodontic wires have primarily concentrated on corrosion and the generation of corrosion byproducts (ions). According to several studies,1,3-6 nickel (Ni), iron (Fe), chromium (Cr), manganese (Mn), and nickel from nickel-titanium alloy are the main corrosion products for stainless steel and titanium alloys. On the other hand, fixed braces with wires interfere with thorough cleaning methods, encourage plaque build-up, and exacerbate tooth discoloration.7 The effectiveness of air-polishing systems in removing dental plaque has been widely studied and documented. These systems utilize abrasive particles such as calcium, sodium, silicate phosphor, sodium bicarbonate, or calcium carbonate to remove plaque from the teeth effectively. In addition to their ability to remove plaque, these systems also release controlled air and water jets, further aiding in plaque removal. Compared to traditional professional dental prophylaxis (PDP), air-polishing systems are more effective in removing plaque and require less working time and operator effort, making them an attractive option for patients and dental professionals.8,9
Previous studies have shown that air polishing is the most effective and successful method for removing plaque around orthodontic brackets and archwires.10 This is because air polishing is more effective at eliminating stain and plaque deposits than traditional scaling and rubber cup polishing. With air polishing, the dental expert can remove stains quickly and with less effort. It has been demonstrated that using air polishers on surfaces made of enamel is safe and does not cause the enamel to slough subsequently.11
Air polishing may cause gingival bleeding and abrasion, but clinically, these effects are irrelevant because they are transient.11 In addition to removing plaque, air polishing can also help prevent corrosion and breakdown of materials in orthodontic appliances caused by the aggressive electrolytic environment in the human mouth.12 The wet environment in the oral cavity contributes to electrolytic or electrochemical corrosion, which can compromise the integrity of orthodontic attachments over time.
A surface oxide layer is created when the surface of some metals reacts with oxygen, preventing an attacking chemical from accessing the metal surface. When a metal is shielded from the elements, its ability to corrode depends on the properties of the protective covering. As long as the surface oxide layer is there, metallic materials are resistant to corrosion. However, the oxide layer dissolves when an alloy reaches its breakdown potential, which starts surface corrosion and pitting.13 Due to their imperfect smoothness, orthodontic wires and brackets are the most susceptible to pitting corrosion. They can have a lot of pits when viewed in microscopic detail. Because of their ability to retain bacteria that generate plaque, this property is thought to increase their susceptibility to corrosion. These microorganisms cause localized oxygen deprivation and pH decline, both of which affect the passivation process.14,15
Corrosion is an electrochemical process clinically described as a loss of metal or its transformation into an oxide. Since it is an oxidative reaction, it takes place at the system’s anode. A related cathode reaction is necessary to maintain electroneutrality, and the corrosion process halts if either the anode reaction or the cathode reaction is hindered. Orthodontic appliance corrosion can have serious clinical effects, such as dimension loss that results in less force being applied to the teeth or stress corrosion failure of the appliance.16 The clinical significance of corrosion is numerous.17 Firstly, corrosion increases the frictional force at the archwire‒bracket interface by increasing surface roughness. Secondly, local pain or swelling near orthodontic appliances has been linked to corrosion products in the absence of an infection, which can result in a secondary infection. Thirdly, corrosion has a cytotoxic effect and exhibits biological reactions. Lastly, it weakens the appliance. Teflon, epoxy, polymer, and rhodium compounds, among others, are frequently used by manufacturers to coat stainless steel or nickel-titanium wires. The mechanical and frictional characteristics of archwires are likely to be affected by the presence of a coating layer. As a result, the producers always strive to coat the wires with a substance that exhibits ideal visual and frictional properties.18
The present study investigated the effect of air abrasive polishing on nickel release and corrosion of stainless steel and nickel-titanium archwires.
Methods
Sources of Materials
One brand of orthodontic archwire (Ultimate WireTM) was obtained from International Orthodontic Services, Stafford, USA. Stainless steel, coated stainless steel, nickel-titanium, and coated nickel-titanium rectangular archwires of one brand (The Ultimate WireTM) were obtained from IOS (International Orthodontic Services, Stafford, USA).
Experimental Design
The experiment conducted in the context above sought to comprehensively assess the impact of air polishing on stainless steel wires used in orthodontic treatment. By dividing the wire samples into distinct groups based on varying polishing durations (5, 10, and 20 seconds) alongside a control group that underwent no polishing, the researchers could meticulously analyze the outcomes (Figure 1). Employing state-of-the-art equipment in the form of Prophy-Mate Neo flash pearl calcium carbonate airborne particles and Prophy-Mate Neo polishing tools from NSK Co.19

Figure 1.
A customized holding device for air polishing, length of wire used, CNC block with wire attached to it, and archwires used
.
A customized holding device for air polishing, length of wire used, CNC block with wire attached to it, and archwires used
This study’s experimental setup involved using a specialized holding mechanism equipped with brackets to secure the wires for air polishing. Following the air abrasion process, the wires were meticulously extracted from the CNC block using a Wingert plier and subjected to a brief immersion in an ethanol-filled ultrasonic machine to eliminate residual calcium carbonate particles.20 Subsequently, the wires were introduced into vacuum-glass tubes containing 10 mL of synthetic saliva with a pH of 6.75. These tubes were securely sealed and placed within a controlled incubator environment at 37 °C for 28 days.21
The wires were swiftly relocated to another tube filled with 10 mL of artificial saliva in seven days. Once again, after another seven days, the wires found a new home in a fresh tube, still containing 10 mL of artificial saliva. This meticulous process adhered to the esteemed ISO/IEC 17025:2005 standards. The primary objective of this study was to delve into the intricate relationship between polishing times and the resulting surface roughness and microhardness of stainless steel wires used in orthodontic treatment. The findings of this investigation hold immense value for clinicians, as they shed light on the optimal air polishing parameters that can lead to enhanced treatment outcomes and heightened patient comfort. With this knowledge, clinicians can revolutionize their approach, offering patients a remarkable orthodontic experience.
Atomic absorption spectrophotometric analysis
After abrasion polishing, each wire was placed individually in a plain tube containing 10 mL of artificial saliva. The samples were examined after the incubation period (7, 14, and 28 days). For calculating the Ni ion release, the technician transferred the artificial saliva to a spectrophotometer, and the quantitative analysis of nickel was performed using a flam ASS with a wavelength of 341.5 nm. The technician calculated the results according to the calibration curve of the atomic absorption spectrophotometer. The release of Ni ions at 7, 14, and 28 days was evaluated using the atomic absorption spectrophotometer.
Scanning electron microscopy analysis
Analysis of the surface micromorphology of the archwires was conducted using scanning electron microscopy (SEM).
Statistical analysis
In this study, ANOVA was used to compare the mean survival times of the three test groups. The least significant difference (LSD) test is a post hoc test often used after ANOVA to determine which pairwise comparisons are significant. In this study, the LSD test was used to determine which pairs of group means were significantly different from each other. The significance level was determined at P < 0.05, meaning any results with a P-value less than 0.05 were considered statistically significant.
Results
The results showed a highly significant (P ≤ 0.05) increase in the amount of Ni2+ released during the study period, coincident with an increase in polishing time. Compared to the other archwires, coated stainless steel showed minimum release types (Tables 1, 2, and 3). Before and during the application of calcium carbonate air abrasive polishing, the surface micromorphology of the archwires was analyzed and observed using SEM at a magnification of × 2000. It was discovered that the modification of the surface of the tested archwire increased with the application of air-abrasive polishing (Table 4). This was demonstrated by the emergence of multiple corroded pits of various sizes and depths concurrent with increased polishing time, as presented in Figure 2.
Table 1.
Accumulative Ni ion release from different arch wire types at different polishing times (incubation period 7 days)
Time of
polishing
|
Types of wire
|
Mean
(μg/dL)
|
SD
|
Min
|
Max
|
F-test
|
P
value
|
| Control |
Coated stainless steel |
17.90817 |
0.103529 |
17.812 |
18.004 |
160.002 |
0.0001 |
| Stainless steel |
18.83367 |
0.219093 |
18.632 |
19.035 |
| Coated nickel titanium |
20.22817 |
0.191160 |
20.052 |
20.404 |
| Nickel titanium |
20.44567 |
0.347259 |
20.127 |
20.764 |
| 5 s |
Coated stainless steel |
18.13317 |
0.066289 |
18.071 |
18.195 |
122.225 |
0.0001 |
| Stainless steel |
19.23267 |
0.241002 |
19.011 |
19.454 |
| Coated nickel titanium |
20.24017 |
0.387242 |
19.885 |
20.595 |
| Nickel titanium |
20.63250 |
0.182762 |
20.464 |
20.801 |
| 10 s |
Coated stainless steel |
18.09067 |
0.111744 |
17.987 |
18.194 |
253.798 |
0.0001 |
| Stainless steel |
19.17083 |
0.194859 |
18.988 |
19.350 |
| Coated nickel titanium |
20.40067 |
0.315491 |
20.111 |
20.690 |
| Nickel titanium |
20.89367 |
0.019765 |
20.874 |
20.913 |
| 20 s |
Coated stainless steel |
19.59617 |
0.106172 |
19.498 |
19.701 |
305.901 |
0.0001 |
| Stainless steel |
19.31717 |
0.201019 |
19.132 |
19.502 |
| Coated nickel titanium |
20.70950 |
0.026539 |
20.683 |
20.735 |
| Nickel titanium |
21.04983 |
0.055708 |
20.997 |
21.102 |
Table 2.
Accumulative Ni ion release from different arch wire types at different polishing times (incubation period14 days)
Time of
polishing
|
Types of wire
|
Mean
(μg/dL)
|
SD
|
Min
|
Max
|
F-test
|
P
value
|
| Control |
Coated stainless steel |
18.02633 |
0.005502 |
18.021 |
18.032 |
387013.265 |
0.0001 |
| Stainless steel |
18.75033 |
0.005502 |
18.745 |
18.756 |
| Coated nickel titanium |
20.68633 |
0.005502 |
20.681 |
20.692 |
| Nickel titanium |
20.81733 |
0.005502 |
20.812 |
20.823 |
| 5 s |
Coated stainless steel |
18.81633 |
0.005502 |
18.811 |
18.822 |
222723.308 |
0.0001 |
| Stainless steel |
18.79100 |
0.007183 |
18.780 |
18.799 |
| Coated nickel titanium |
20.53933 |
0.005502 |
20.534 |
20.545 |
| Nickel titanium |
20.99400 |
0.005477 |
20.989 |
20.999 |
| 10 s |
Coated stainless steel |
19.46133 |
0.005502 |
19.456 |
19.467 |
266710.887 |
0.0001 |
| Stainless steel |
19.20633 |
0.005502 |
19.201 |
19.212 |
| Coated nickel titanium |
21.17233 |
0.005502 |
21.167 |
21.178 |
| Nickel titanium |
21.47433 |
0.005502 |
21.469 |
21.480 |
| 20 s |
Coated stainless steel |
19.83733 |
0.005502 |
19.832 |
19.843 |
249183.618 |
0.0001 |
| Stainless steel |
19.69133 |
0.005502 |
19.686 |
19.697 |
| Coated nickel titanium |
21.58333 |
0.005502 |
21.578 |
21.589 |
| Nickel titanium |
21.81033 |
0.005502 |
21.805 |
21.816 |
Table 3.
Accumulative Ni ion release from different arch wire types at different polishing times (incubation period 28 days)
Time of
polishing
|
Types of wire
|
Mean
(μg/dL)
|
SD
|
Min
|
Max
|
F-test
|
P
value
|
| Control |
Coated stainless steel |
20.14467 |
0.004502 |
20.139 |
20.151 |
553229.527 |
0.0001 |
| Stainless steel |
20.16067 |
0.004502 |
20.155 |
20.167 |
| Coated nickel titanium |
22.56550 |
0.004764 |
22.559 |
22.572 |
| Nickel titanium |
22.54567 |
0.004502 |
22.540 |
22.552 |
| 5 s |
Coated stainless steel |
20.61667 |
0.004502 |
20.611 |
20.623 |
2508031.418 |
0.0001 |
| Stainless steel |
20.63767 |
0.004502 |
20.632 |
20.644 |
| Coated nickel titanium |
25.75167 |
0.004502 |
25.746 |
25.758 |
| Nickel titanium |
25.35100 |
0.004099 |
25.346 |
25.357 |
| 10 s |
Coated stainless steel |
20.86167 |
0.004502 |
20.856 |
20.868 |
3682318.034 |
0.0001 |
| Stainless steel |
20.76167 |
0.004502 |
20.756 |
20.768 |
| Coated nickel titanium |
26.55100 |
0.004099 |
26.546 |
26.557 |
| Nickel titanium |
26.75100 |
0.004099 |
26.746 |
26.757 |
| 20 s |
Coated stainless steel |
21.53767 |
0.004502 |
21.532 |
21.544 |
3946823.152 |
0.0001 |
| Stainless steel |
21.53800 |
0.004050 |
21.534 |
21.544 |
| Coated nickel titanium |
27.60767 |
0.004502 |
27.602 |
27.614 |
| Nickel titanium |
27.80767 |
0.004502 |
27.802 |
27.814 |
Table 4.
Comparison between the mean values of Ni ions released for all different arch wire types at different polishing time
|
Incubation periods
|
Time of polishing (s)
|
Types of wire
|
Mean difference
|
P
value
|
| 7 days |
Control |
Stainless steel |
Coated stainless |
-0.925500* |
0.000 |
| Nickel titanium |
-2.320000* |
0.000 |
| Coated nickel titanium |
-2.537500* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.394500* |
0.000 |
| Coated nickel titanium |
-1.612000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.217500 |
0.012 |
| 5 s |
Stainless steel |
Coated stainless |
-1.099500* |
0.000 |
| Nickel titanium |
-2.107000* |
0.000 |
| Coated nickel titanium |
-2.499333* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.007500* |
0.000 |
| Coated nickel titanium |
-1.399833* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.392333* |
0.013 |
| 10 s |
Stainless steel |
Coated stainless |
-1.080167* |
0.000 |
| Nickel titanium |
-2.310000* |
0.000 |
| Coated nickel titanium |
-2.803000* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.229833* |
0.000 |
| Coated nickel titanium |
-1.722833* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.493000* |
0.000 |
| 20 s |
Stainless steel |
Coated stainless |
0.279000* |
0.001 |
| Nickel titanium |
-1.113333* |
0.000 |
| Coated nickel titanium |
-1.453667* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.392333* |
0.000 |
| Coated nickel titanium |
-1.732667* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.340333* |
0.000 |
| 14 days |
Control |
Stainless steel |
Coated stainless |
-0.724000* |
0.000 |
| Nickel titanium |
-2.660000* |
0.000 |
| Coated nickel titanium |
-2.791000* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.936000* |
0.000 |
| Coated nickel titanium |
-2.067000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.131000* |
0.000 |
| 5 s |
Stainless steel |
Coated stainless |
0.025333* |
0.000 |
| Nickel titanium |
-1.723000* |
0.000 |
| Coated nickel titanium |
-2.177667* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.748333* |
0.000 |
| Coated nickel titanium |
-2.203000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.454667* |
0.000 |
| 10 s |
Stainless steel |
Coated stainless |
0.255000* |
0.000 |
| Nickel titanium |
-1.711000* |
0.000 |
| Coated nickel titanium |
-2.013000* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.966000* |
0.000 |
| Coated nickel titanium |
-2.268000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.302000* |
0.000 |
| 20 s |
Stainless steel |
Coated stainless |
0.146000* |
0.000 |
| Nickel titanium |
-1.746000* |
0.000 |
| Coated nickel titanium |
-1.973000* |
0.000 |
| Coated stainless |
Nickel titanium |
-1.892000* |
0.000 |
| Coated nickel titanium |
-2.119000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.227000* |
0.000 |
| 28 days |
Control |
Stainless steel |
Coated stainless |
-0.016000* |
0.000 |
| Nickel titanium |
-2.420833* |
0.000 |
| Coated nickel titanium |
-2.401000* |
0.000 |
| Coated stainless |
Nickel titanium |
-2.404833* |
0.000 |
| Coated nickel titanium |
-2.385000* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
0.019833* |
0.000 |
| 5 s |
Stainless steel |
Coated stainless |
-0.021000* |
0.000 |
| Nickel titanium |
-5.135000* |
0.000 |
| Coated nickel titanium |
-4.734333* |
0.000 |
| Coated stainless |
Nickel titanium |
-5.114000* |
0.000 |
| Coated nickel titanium |
-4.713333* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
0.400667* |
0.000 |
| 10 s |
Stainless steel |
Coated stainless |
0.100000* |
0.000 |
| Nickel titanium |
-5.689333* |
0.000 |
| Coated nickel titanium |
-5.889333* |
0.000 |
| Coated stainless |
Nickel titanium |
-5.789333* |
0.000 |
| Coated nickel titanium |
-5.989333* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.200000* |
0.000 |
| 20 s |
Stainless steel |
Coated stainless |
-0.000333 |
0.000 |
| Nickel titanium |
-6.070000* |
0.000 |
| Coated nickel titanium |
-6.270000* |
0.000 |
| Coated stainless |
Nickel titanium |
-6.069667* |
0.000 |
| Coated nickel titanium |
-6.269667* |
0.000 |
| Nickel titanium |
Coated nickel titanium |
-0.200000* |
0.000 |
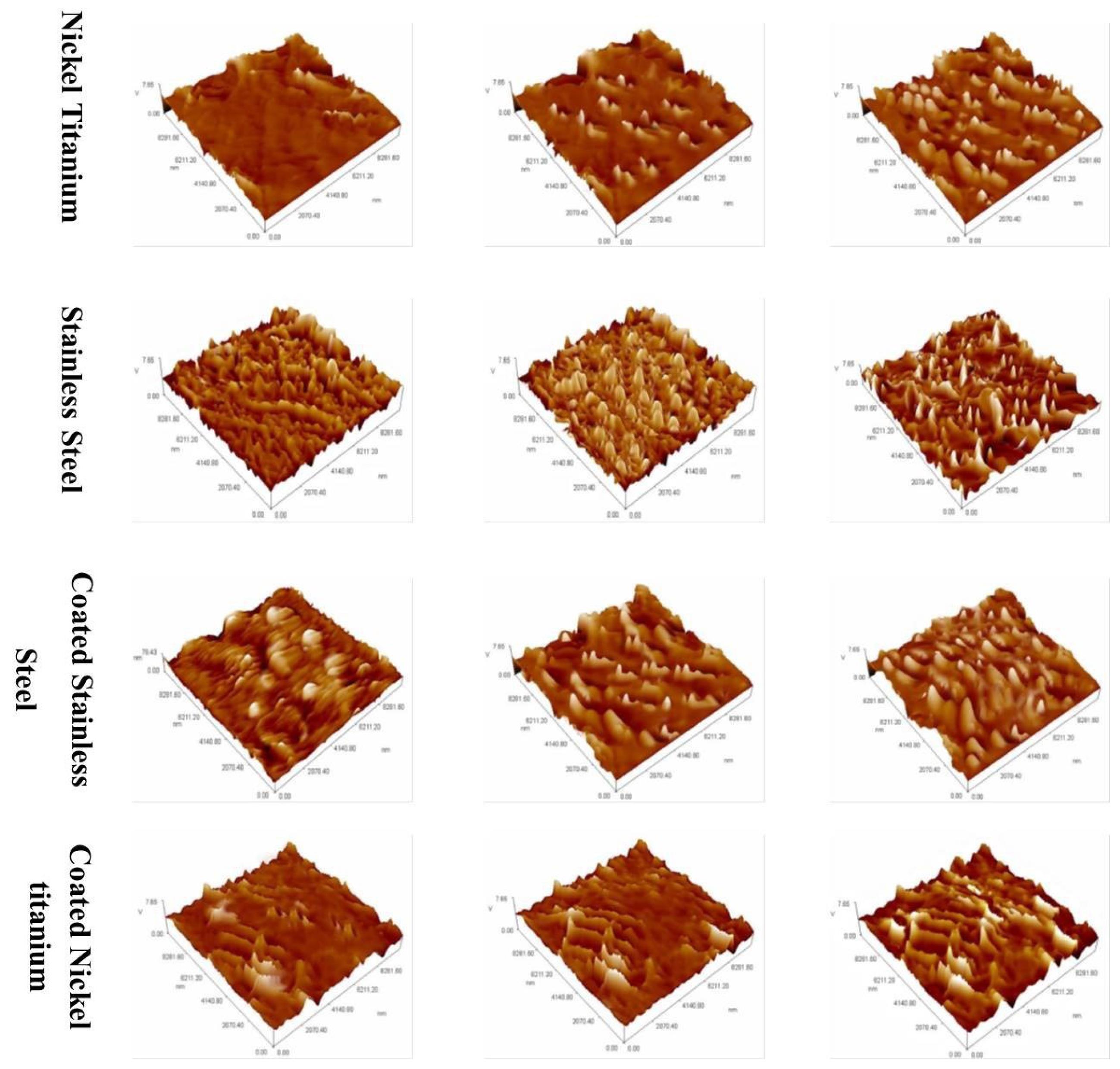
Figure 2.
Surface micromorphology of each type of archwires at × 2000 magnification using SEM. A represents the surface of control arch wires to air polishing; B represents the surface of the arch wires after 5 seconds of air polishing; C represents the surface of archwires after 20 seconds of air polishing
.
Surface micromorphology of each type of archwires at × 2000 magnification using SEM. A represents the surface of control arch wires to air polishing; B represents the surface of the arch wires after 5 seconds of air polishing; C represents the surface of archwires after 20 seconds of air polishing
Discussion
Adult patients in need of orthodontic care are becoming more prevalent today. The eating habits of this group of patients were different from those of adolescents in that they consumed more colored liquids, such as coffee and tea, which stain the enamel and leave a deposit, necessitating a cleaning procedure.22 In the past, using a rubber cup or brush to apply an abrasive paste to teeth during PDP has been the standard procedure for polishing teeth. This procedure can be used to get rid of supragingival plaque and stains. However, it is difficult, time-consuming, and ineffective to remove supragingival deposits and stains from the region around bonded orthodontic appliances using a rubber cup and abrasive paste.23 Therefore, it can be deduced that airflow polishing offers advantages over traditional PDP despite its inability to effectively remove dental plaque and discoloration due to its encouragement of reduced working time and operator effort. Furthermore, this approach has been widely employed to address adult patients’ compromised orthodontic treatment compliance and treatment satisfaction by removing tooth discoloration.8,9 Dental alloys can release metal ions into the oral cavity due to corrosion processes, even though they have a protective oxide coating on the metal surface.24 The Ni2+ released from archwires after air polishing has not been previously studied. To demonstrate and emphasize how air polishing affects the Ni2+ release in synthetic saliva, this study performed three separate air polishing sessions using a calcium carbonate powder.24-26
Previous studies on metal ions released from orthodontic archwires have found that the corrosion process can take up to four weeks to complete, suggesting that corrosion is a slow process and that it may take an extended period for the release of metal ions to reach its maximum potential. In this study, the incubation period for the archwires in synthetic saliva was set at 28 days, equivalent to approximately four weeks.27,28 The increase in Ni2+ release that happens simultaneously with an increase in polishing time may be due to the rise in the surface roughness of archwires, increasing the surface area of the wire. Longer polishing durations were predicted to lead to an increase in surface area and texture roughness.20 In addition, when the surface roughness increases, the surface area that comes into contact with the saliva increases, thereby increasing the Ni2+ released.29 According to Pakshir et al30 in 2011 and Roberge31 in 2012, when manufacturers create pits, the passive layer is locally dissolved, and the pit’s depth in the underlying metal increases quickly.30,31 As a result, these surface irregularities accelerate the corrosion process. More ions were produced and detected due to the development of an electrochemical cell in which the cathode is a sizable region of passive metal, and a very small area of active metal is the anode.30,31 In addition, it was reported that a passive layer rich in chromium and typically 3‒5 nm in depth, or around 15 layers of atoms, gives the material its corrosion-resistant feature.32
An oxidation-reduction reaction produces the passive layer, during which the passivating substance is reduced, and chromium and nickel are oxidized. Rapid general and/or galvanic corrosion may result if this layer is not permitted to form or if it is damaged.32 The stainless steel and titanium alloys used in orthodontic appliances prevent corrosion by forming a passive surface oxide coating. The barrier is vulnerable to mechanical and chemical damage; hence, it is not impenetrable. SEM studies of archwires made of stainless steel and Ni-Ti subjected to electrochemical corrosion in artificial saliva by air prophy revealed pitting corrosion on the wire surface.17
The polishing process of abrasive particles damages the chromium oxide layer, a protective passive layer that is likely destroyed, exposing the fresh metal to corrosion and speeding up surface degradation.33,34 The total amount of Ni2+ in each of the four major groups was lower than their combined daily intake. The results of the present study showed that the adult acceptable upper intake threshold for nickel is 45 mg/day, indicating that all discharged ions present following the air polishing professional cleaning method were below daily maximums and hazardous values.35,36 Metallic orthodontic appliances may release metal ions due to corrosion in the oral cavity. Airflow polishing may impact the ion release from orthodontic wires.37
Conclusion
The results of this study provide valuable insights into the potential use of calcium carbonate air polishing during orthodontic treatment. This technique can be effectively applied by adhering to the recommended polishing time of 5 seconds and implementing longer polishing pauses for adults. Furthermore, this investigation sheds light on the impact of air-powder polishing on surface roughness and topography, particularly focusing on orthodontic archwires. Notably, the use of calcium bicarbonate powder in this method has been found to alter the surface roughness and topography of the archwires, a finding that has not been previously explored in vitro. As a practical recommendation, orthodontists should consider employing new archwires after air-powder polishing, particularly when performing tooth movements requiring reduced friction. By incorporating these findings into clinical practice, orthodontists can enhance treatment outcomes and optimize patient care.
Acknowledgments
The authors are grateful to the College of Dentistry/ University of Mosul for providing facilities to accomplish this work.
Competing Interests
The authors declare no competing interests.
Ethical Approval
College of Dentistry/University of Baghdad (UoB/CoD 600 on 10.04.2022).
Funding
Self-funded.
References
- Hameed HM, Al-Groosh DH. Effects of air abrasive polishing on iron ion release from different metal self-ligating orthodontic brackets. Health Sci 2018; 7(9):166-72. [ Google Scholar]
- Patel R, Bhanat S, Patel D, Shah B. Corrosion inhibitory ability of Ocimum sanctum Linn (Tulsi) rinse on ion release from orthodontic brackets in some mouthwashes: an invitro study. Natl J Community Med 2014; 5(1):135-9. [ Google Scholar]
- Veien NK, Borchorst E, Hattel T, Laurberg G. Stomatitis or systemically-induced contact dermatitis from metal wire in orthodontic materials. Contact Dermatitis 1994; 30(4):210-3. doi: 10.1111/j.1600-0536.1994.tb00645.x [Crossref] [ Google Scholar]
- Ramadan AA. Effect of nickel and chromium on gingival tissues during orthodontic treatment: a longitudinal study. World J Orthod 2004; 5(3):230-4. [ Google Scholar]
- Schultz JC, Connelly E, Glesne L, Warshaw EM. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis 2004; 15(3):154-7. doi: 10.2310/6620.2004.04022 [Crossref] [ Google Scholar]
- Menezes LM, Quintão CA, Bolognese AM. Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofacial Orthop 2007; 131(5):635-8. doi: 10.1016/j.ajodo.2005.07.022 [Crossref] [ Google Scholar]
- Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod 1982; 81(2):93-8. doi: 10.1016/0002-9416(82)90032-x [Crossref] [ Google Scholar]
- Hosoya Y, Johnston JW. Evaluation of various cleaning and polishing methods on primary enamel. J Pedod 1989; 13(3):253-69. [ Google Scholar]
- Prathap S, Rajesh H, Boloor VA, Rao AS. Extrinsic stains and management: a new insight. J Acad Indus Res 2013; 1(8):435-42. [ Google Scholar]
- Barnes CM, Russell CM, Gerbo LR, Wells BR, Barnes DW. Effects of an air-powder polishing system on orthodontically bracketed and banded teeth. Am J Orthod Dentofacial Orthop 1990; 97(1):74-81. doi: 10.1016/s0889-5406(05)81712-3 [Crossref] [ Google Scholar]
- Hassan AR, Mohammed SA. Evaluating the effect of air abrasive polishing on friction and surface micromorphology of ceramic brackets using different wires. Indian J Forensic Med Toxicol 2021; 15(4):400-7. [ Google Scholar]
- Maijer R, Smith DC. Biodegradation of the orthodontic bracket system. Am J Orthod Dentofacial Orthop 1986; 90(3):195-8. doi: 10.1016/0889-5406(86)90065-x [Crossref] [ Google Scholar]
- Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod 1999; 69(1):39-44. doi: 10.1043/0003-3219(1999)069<0039:cossnt>2.3.co;2 [Crossref] [ Google Scholar]
- House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ. Corrosion of orthodontic appliances--should we care?. Am J Orthod Dentofacial Orthop 2008; 133(4):584-92. doi: 10.1016/j.ajodo.2007.03.021 [Crossref] [ Google Scholar]
- Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod 2002; 72(3):222-37. doi: 10.1043/0003-3219(2002)072<0222:ivaooa>2.0.co;2 [Crossref] [ Google Scholar]
- Toms AP. The corrosion of orthodontic wire. Eur J Orthod 1988; 10(2):87-97. doi: 10.1093/ejo/10.2.87 [Crossref] [ Google Scholar]
- Pulikkottil VJ, Chidambaram S, Bejoy PU, Femin PK, Paul P, Rishad M. Corrosion resistance of stainless steel, nickel-titanium, titanium molybdenum alloy, and ion-implanted titanium molybdenum alloy archwires in acidic fluoride-containing artificial saliva: an in vitro study. J Pharm Bioallied Sci 2016; 8(Suppl 1):S96-S9. doi: 10.4103/0975-7406.192032 [Crossref] [ Google Scholar]
- Abbas AA, Alhuwaizi AF. The effect of wire dimension, type and thickness of coating layer on friction of coated stainless-steel arch wires. Int J Med Res Health Sci 2018; 7(3):115-21. [ Google Scholar]
- Hameed HM, Al-Groosh DH. Effects of air abrasive polishing on chromium ion release from different metal self-ligating orthodontic brackets. MDJ 2019; 16(1):60-7. [ Google Scholar]
- Parmagnani EA, Basting RT. Effect of sodium bicarbonate air abrasive polishing on attrition and surface micromorphology of ceramic and stainless steel brackets. Angle Orthod 2012; 82(2):351-62. doi: 10.2319/040111-235.1 [Crossref] [ Google Scholar]
- Chaturvedi TP, Upadhayay SN. An overview of orthodontic material degradation in oral cavity. Indian J Dent Res 2010; 21(2):275-84. doi: 10.4103/0970-9290.66648 [Crossref] [ Google Scholar]
- Veličković M, Sekulic Markovic S, Acovic A, Radovanovic S, Kanjevac T. Non-invasive treatment of multiple enamel hypoplasia: a case report. Med Čas 2021; 55(4):144-7. doi: 10.5937/mckg55-25392 [Crossref] [ Google Scholar]
- Barnes CM, Covey D, Watanabe H, Simetich B, Schulte JR, Chen H. An in vitro comparison of the effects of various air polishing powders on enamel and selected esthetic restorative materials. J Clin Dent 2014; 25(4):76-87. [ Google Scholar]
- Craig RG, Peyton FA. The micro-hardness of enamel and dentin. J Dent Res 1958; 37(4):661-8. doi: 10.1177/00220345580370041301 [Crossref] [ Google Scholar]
- Tsujikawa M, Noguchi S, Yamauchi N, Ueda N, Sone T. Effect of molybdenum on hardness of low-temperature plasma carburized austenitic stainless steel. Surf Coat Technol 2007; 201(9-11):5102-7. doi: 10.1016/j.surfcoat.2006.07.127 [Crossref] [ Google Scholar]
- Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent 2008; 36(1):74-9. doi: 10.1016/j.jdent.2007.10.008 [Crossref] [ Google Scholar]
- Gürsoy S, Acar AG, Seşen C. Comparison of metal release from new and recycled bracket-archwire combinations. Angle Orthod 2005; 75(1):92-4. doi: 10.1043/0003-3219(2005)075<0092:comrfn>2.0.co;2 [Crossref] [ Google Scholar]
- Dos Santos AA, Pithon MM, Carlo FG, Carlo HL, de Lima BA, Dos Passos TA. Effect of time and pH on physical-chemical properties of orthodontic brackets and wires. Angle Orthod 2015; 85(2):298-304. doi: 10.2319/032914-234.1 [Crossref] [ Google Scholar]
- Fontana MG. Corrosion Engineering. McGraw-Hill Education; 2005.
- Pakshir M, Bagheri T, Kazemi MR. In vitro evaluation of the electrochemical behaviour of stainless steel and Ni-Ti orthodontic archwires at different temperatures. Eur J Orthod 2013; 35(4):407-13. doi: 10.1093/ejo/cjr055 [Crossref] [ Google Scholar]
- Roberge PR. Handbook of Corrosion Engineering. McGraw-Hill; 2000.
- Kerber SJ, Tverberg J. Stainless steel. Adv Mater Process 2000; 158(5):33-6. [ Google Scholar]
- Marcus P. Corrosion Mechanisms in Theory and Practice. CRC Press; 2011.
- Revie RW. Uhlig’s Corrosion Handbook. John Wiley & Sons; 2011.
- Khamees AM, Al-Joubori SK. Comparison of metal ions release and corrosion potential from different bracket archwire combinations (an in vitro study). J Baghdad Coll Dent 2014; 26(1):171-9. [ Google Scholar]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 2001; 101(3):294-301. doi: 10.1016/s0002-8223(01)00078-5 [Crossref] [ Google Scholar]
- Rafeeq RA, Saleem AI, Nissan LM. Ions release from fixed orthodontic appliance in two different mouthwashes. J Baghdad Coll Dent 2014; 26(4):152-5. [ Google Scholar]